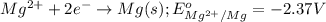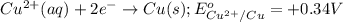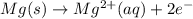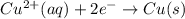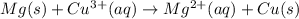Chemistry, 18.06.2021 06:10 angscott9638
I neeed help nowww HELP NOOW
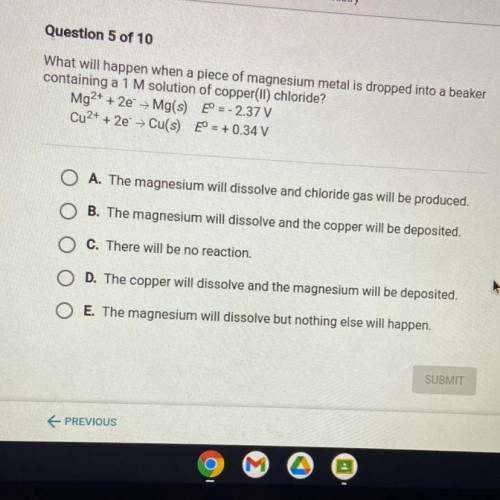
What will happen when a piece of magnesium metal is dropped into a beaker
containing a 1 M solution of copper(II) chloride?
Mg2+ + 2e + Mg(s) eº = -2.37 V
Cu2+ + 2e → Cu(s) E° = + 0.34 V

Answers: 1


Another question on Chemistry



Chemistry, 22.06.2019 19:20
For a research project, a student decided to test the effect of the lead(ii) ion (pb2+) on the ability of salmon eggs to hatch. this ion was obtainable from the water‐soluble salt, lead(ii) nitrate, which the student decided to make by the following reaction. pbo(s) + 2 hno3(aq) → pb(no3)2(aq) + h2o losses of product for various reasons were expected, and a yield of 86.0% was expected. in order to have 5.00 g of product at this yield, how many grams of pbo should be reacted? (assume that sufficient nitric acid, hno3, would be used.)
Answers: 1

Chemistry, 22.06.2019 19:30
Astudent conducts an experiment to determine how the amount of water given to a plant affects its growth. what is the independent variable for this experiment?
Answers: 1
You know the right answer?
I neeed help nowww HELP NOOW
What will happen when a piece of magnesium metal is dropped into a bea...
Questions

Mathematics, 26.04.2021 21:40


Arts, 26.04.2021 21:40

Biology, 26.04.2021 21:40


Computers and Technology, 26.04.2021 21:40

Mathematics, 26.04.2021 21:40

Mathematics, 26.04.2021 21:40

Mathematics, 26.04.2021 21:40

Mathematics, 26.04.2021 21:40









Social Studies, 26.04.2021 21:40

Mathematics, 26.04.2021 21:40