
Chemistry, 18.06.2021 22:00 davisearron
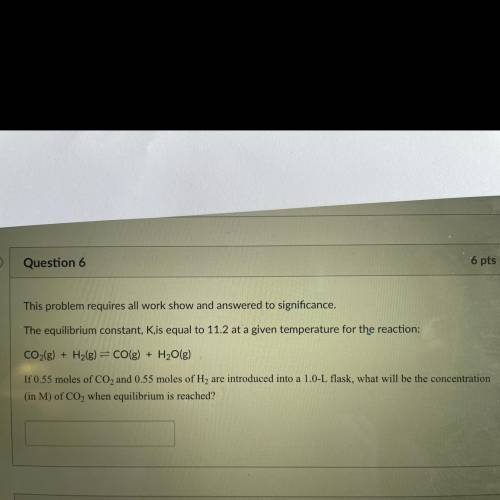
This problem requires all work show and answered to significance.
The equilibrium constant, Kis equal to 11.2 at a given temperature for the reaction:
CO2(g) + H2(g) = CO(g) + H2O(g)
If 0.55 moles of CO2 and 0.55 moles of H2 are introduced into a 1.0-L flask, what will be the concentration
(in M) of CO2 when equilibrium is reached?

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 11:40
Which type of precipitation would most likely form when the surface air temperature is slightly below freezing and the air temperature increases as you move upward away from the ground?
Answers: 2

Chemistry, 22.06.2019 14:00
Which of the following is true about a carbonated soft drink? . the carbon dioxide is the solvent, and water is the solute.. the water is the solution, and carbon dioxide is the solvent.. the carbon dioxide is the solution, and the water is the solvent.. the water is the solvent, and the carbon dioxide is the solute.. .
Answers: 1

Chemistry, 22.06.2019 17:00
Complete each row of the table below by filling in the missing prefix or missing exponent.
Answers: 1
You know the right answer?
This problem requires all work show and answered to significance.
The equilibrium constant, Kis equ...
Questions



History, 12.12.2020 16:40

Mathematics, 12.12.2020 16:40

Mathematics, 12.12.2020 16:40


History, 12.12.2020 16:40




Mathematics, 12.12.2020 16:40

Mathematics, 12.12.2020 16:40

English, 12.12.2020 16:40

Mathematics, 12.12.2020 16:40

Mathematics, 12.12.2020 16:40

Mathematics, 12.12.2020 16:40

Mathematics, 12.12.2020 16:40

Mathematics, 12.12.2020 16:40

SAT, 12.12.2020 16:40

Mathematics, 12.12.2020 16:40



