
Chemistry, 18.06.2021 22:30 rosenatalie222
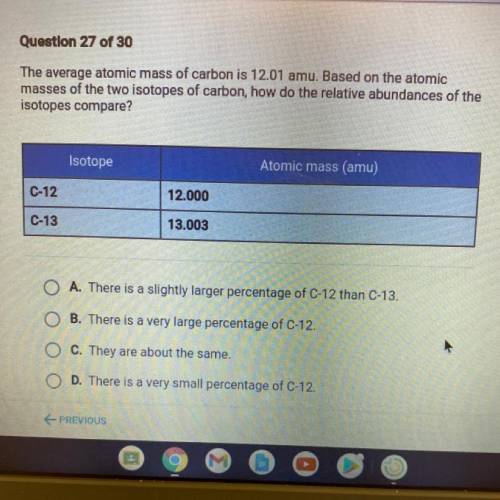
PLS HELP The average atomic mass of carbon is 12.01 amu. Based on the atomic
masses of the two isotopes of carbon, how do the relative abundances of the
isotopes compare?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 17:00
What were the success and failures that came to boyle’s excitements?
Answers: 1

Chemistry, 21.06.2019 23:30
For the following dehydrohalogenation (e2) reaction, draw the zaitsev product(s) resulting from elimination involving c3–c4 (i.e., the carbon atoms depicted with stereobonds). show the product stereochemistry clearly. if there is more than one organic product, both products may be drawn in the same box. ignore elimination involving c3 or c4 and any carbon atom other than c4 or c3.
Answers: 3

Chemistry, 22.06.2019 14:50
Which of the following is most likely true about water in chemical solutions?
Answers: 1

Chemistry, 22.06.2019 21:00
What is the chemical formula for the compound formed between sodium and flour one
Answers: 1
You know the right answer?
PLS HELP The average atomic mass of carbon is 12.01 amu. Based on the atomic
masses of the two isot...
Questions

Mathematics, 01.02.2021 22:40

Mathematics, 01.02.2021 22:40

Mathematics, 01.02.2021 22:40


Mathematics, 01.02.2021 22:40

Mathematics, 01.02.2021 22:40


Mathematics, 01.02.2021 22:40





Mathematics, 01.02.2021 22:40



Mathematics, 01.02.2021 22:40


Health, 01.02.2021 22:40

Biology, 01.02.2021 22:40

Geography, 01.02.2021 22:40




