Chemistry, 18.06.2021 23:50 tonydeanfbg8706
g When aqueous solutions of and are mixed, a solid forms. Determine the mass of solid formed when 140.7 mL of 0.1000 M is mixed with an excess of an aqueous solution of .

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 15:30
Two metal blocks that have slightly different temperatures are placed next to one another. after five minutes, they both have lower but equal temperatures. according to the law of conservation of energy, what most likelyhappened? energy was created inside the blocks.energy was destroyed inside the blocks.energy was absorbed into the blocks from outside the system.energy was transferred from the warmer block to the cooler block.
Answers: 2

Chemistry, 22.06.2019 22:30
Draw the aromatic compound toluene (methylbenzene). show all hydrogen atoms, including those on the ring.
Answers: 1

Chemistry, 23.06.2019 13:30
1. what is boyle’s law? • state the definition of the law in words. • what are the assumptions of boyle’s law? • write at least one mathematical equation that represents the law. • what can be calculated with boyle’s law? • using a gas-filled balloon as an example, describe what is happening to the gas molecules inside the balloon before and after you squeeze it.
Answers: 2

Chemistry, 23.06.2019 14:00
Aracehorse with a kinetic energy of 78,750 j is clocked at 17 m/s when it crosses the finish line. what is the horse's mass?
Answers: 2
You know the right answer?
g When aqueous solutions of and are mixed, a solid forms. Determine the mass of solid formed when 14...
Questions

English, 04.09.2020 01:01


Mathematics, 04.09.2020 01:01


SAT, 04.09.2020 01:01

Biology, 04.09.2020 01:01



History, 04.09.2020 01:01

Mathematics, 04.09.2020 01:01

Biology, 04.09.2020 01:01

Advanced Placement (AP), 04.09.2020 01:01


Mathematics, 04.09.2020 01:01




English, 04.09.2020 01:01


Mathematics, 04.09.2020 01:01

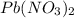 are mixed, a solid forms. Determine the mass of solid formed when 140.7 mL of 0.1000 M NaCl is mixed with an excess of an aqueous solution of
are mixed, a solid forms. Determine the mass of solid formed when 140.7 mL of 0.1000 M NaCl is mixed with an excess of an aqueous solution of  .....(1)
.....(1)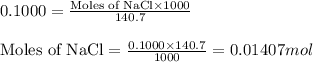
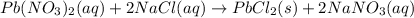
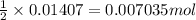 of lead chloride
of lead chloride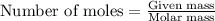 ......(2)
......(2)