Gaseous ethane (CH, CH,) will react with gaseous oxygen (02) to produce gaseous carbon dioxide (CO2) and gaseous water (H,0). Suppose 4.21 g of
ethane is mixed with 31. 9 of oxygen. Calculate the maximum mass of carbon dioxide that could be produced by the chemical reaction. Be sure your answer has
the correct number of significant digits.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:50
Significant figures are digits read directly from the measuring instrument plus one more digit, which is __ by the observer.
Answers: 2

Chemistry, 22.06.2019 07:10
Provide a stepwise curved arrow mechanism that fully explains the outcome of the reaction shown below. oh нао* heat он
Answers: 2

Chemistry, 22.06.2019 17:00
The biosphere of the earth is made up of what compound? organic or inorganic?
Answers: 3

You know the right answer?
Gaseous ethane (CH, CH,) will react with gaseous oxygen (02) to produce gaseous carbon dioxide (CO2)...
Questions

Mathematics, 28.03.2020 22:39

Chemistry, 28.03.2020 22:39



History, 28.03.2020 22:39




Mathematics, 28.03.2020 22:39


Mathematics, 28.03.2020 22:39

Mathematics, 28.03.2020 22:39

English, 28.03.2020 22:39



Biology, 28.03.2020 22:39

Mathematics, 28.03.2020 22:40


History, 28.03.2020 22:40

Mathematics, 28.03.2020 22:40

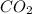 produced is 12.32 g
produced is 12.32 g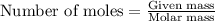 ......(1)
......(1)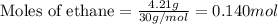
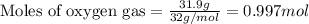
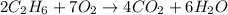
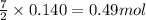 of oxygen gas
of oxygen gas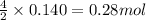 of
of