Liquid hexane
(CH,(CH), CH) will react with gaseous oxygen (0) to produce gaseous carbon dioxide (CO2) and gaseous water (1,0). Suppose 1.72 g
of hexane is mixed with 8.0 g of oxygen. Calculate the maximum mass of water that could be produced by the chemical reaction. Be sure your answer has the
correct number of significant digits.

Answers: 1


Another question on Chemistry

Chemistry, 23.06.2019 04:00
How many liters of water can be produced from 5.0liters of butane gas at stp, assuming excess oxygen? c4h10(g) + 02(g) → co2 (e) + h2o (g)
Answers: 2

Chemistry, 23.06.2019 07:00
Choose the correct statement about licensed veterinarians in the united states. a. they must be certified by the avma. b. they can treat all nonhuman animals. c. they can can treat only animals specified on the license. d. they must choose a specialty.
Answers: 2

Chemistry, 23.06.2019 11:00
Nh4no3 n2o + 2h2o a chemist who is performing this reaction starts with 160.1 g of nh4no3. the molar mass of nh4no3 is 80.03 g/mol; the molar mass of water (h2o) is 18.01 g/mol. what mass, in grams, of h2o is produced?
Answers: 1

Chemistry, 23.06.2019 14:30
The hammering on a train track is often heard twice by workers farther down the track; first as the sound travels through the steel and second as the sound travels through the air. this suggests which graph is true?
Answers: 1
You know the right answer?
Liquid hexane
(CH,(CH), CH) will react with gaseous oxygen (0) to produce gaseous carbon dioxide (C...
Questions


Mathematics, 10.11.2020 18:50

History, 10.11.2020 18:50


Mathematics, 10.11.2020 18:50



Physics, 10.11.2020 18:50

Arts, 10.11.2020 18:50




Physics, 10.11.2020 18:50


Mathematics, 10.11.2020 18:50

Mathematics, 10.11.2020 18:50

Chemistry, 10.11.2020 18:50



Mathematics, 10.11.2020 18:50

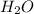 produced is 2.52 g
produced is 2.52 g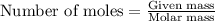 ......(1)
......(1)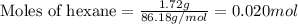
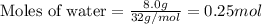
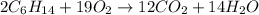
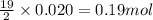 of oxygen gas
of oxygen gas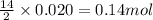 of
of