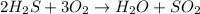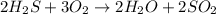A student wrote a chemical equation as shown.
2H₂S+ 302 H₂O + SO₂
What should the stude...

Chemistry, 20.06.2021 07:20 EatsChiken
A student wrote a chemical equation as shown.
2H₂S+ 302 H₂O + SO₂
What should the student do to balance the equation?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:30
Complete the following reactions using word and balanced equations including states. dilute phosphoric acid is added with a calcium hydroxide solution.
Answers: 1

Chemistry, 21.06.2019 22:30
Agas at 155 kpa and standard temperature has an initial volume of 1.00 l. the pressure of the gas rises to 500 kpa as the temperature also rises to 135°c. what is the new volume? 2.16 l 0.463 l 0.207 l 4.82 l
Answers: 3

Chemistry, 22.06.2019 02:00
Which of the following is not a good technique for managing used oil? a) have specific, labeled catch pans available for technicians who are collecting oil b) spills in your shop and any releases on pavement or outside should be poured down a drain c) do not use oil containers for antifreeze or other non-similar fluids d) be prepared for oil spills with the proper absorbents
Answers: 1

Chemistry, 22.06.2019 06:30
The following reaction shows the products when sulfuric acid and aluminum hydroxide react. al(oh)3 + h2so4 → al2(so4)3 + h2o the table shows the calculated amounts of reactants and products when the reaction was conducted in a laboratory. sulfuric acid aluminum hydroxide initial amount of reactant 40 g 15 g theoretical yield of water from reactant 14.69 g 10.38 g what is the approximate amount of the leftover reactant? 11.73 g of sulfuric acid 10.33 g of sulfuric acid 11.12 g of aluminum hydroxide 13.67 g of aluminum hydroxide
Answers: 3
You know the right answer?
Questions

Mathematics, 05.01.2020 17:31

Mathematics, 05.01.2020 17:31


Mathematics, 05.01.2020 17:31

Mathematics, 05.01.2020 17:31




History, 05.01.2020 17:31

Social Studies, 05.01.2020 17:31




History, 05.01.2020 17:31




Social Studies, 05.01.2020 17:31


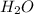 and
and 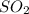 to make the equation balanced.
to make the equation balanced.