
249 g of potassium iodide, KI, is mixed with 496.5 g of lead(II) nitrate, Pb(NO3)2.
The equation of the reaction is represented below.
[Pb(NO3)2 = 331; KI = 166; PbI2 = 461, KNO3 = 101]
Calculate the number of moles of excess reagent left.
Give your answer to three significant figures.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 17:30
Which uses electromagnetic radiation to discover the properties and composition of bodies in space? space probe space station space shuttle space observatory
Answers: 2

Chemistry, 22.06.2019 04:00
Asolution contains 225 g of sodium chloride, nacl, dissolved in enough water to make a 0.25 l of solution. what is the molarity of the solution?
Answers: 2

Chemistry, 22.06.2019 10:10
When electrolyzing copper (ll) chloride, what reaction takes place at the anode? what reaction takes place at the cathode?
Answers: 1

Chemistry, 22.06.2019 20:30
Identify the correct mole ratio for each substance. sodium chloride (nacl) na: cl = 1: ammonium nitrate (nhno) h: o = 4:
Answers: 1
You know the right answer?
249 g of potassium iodide, KI, is mixed with 496.5 g of lead(II) nitrate, Pb(NO3)2.
The equation of...
Questions




Biology, 19.03.2020 06:09


English, 19.03.2020 06:09

Mathematics, 19.03.2020 06:09

Mathematics, 19.03.2020 06:09





History, 19.03.2020 06:10

Mathematics, 19.03.2020 06:10

Physics, 19.03.2020 06:10



Mathematics, 19.03.2020 06:10


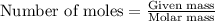 ......(1)
......(1)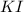 = 249 g
= 249 g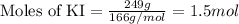
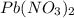 :
: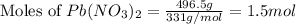
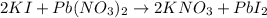
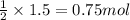 of lead(II) nitrate
of lead(II) nitrate

