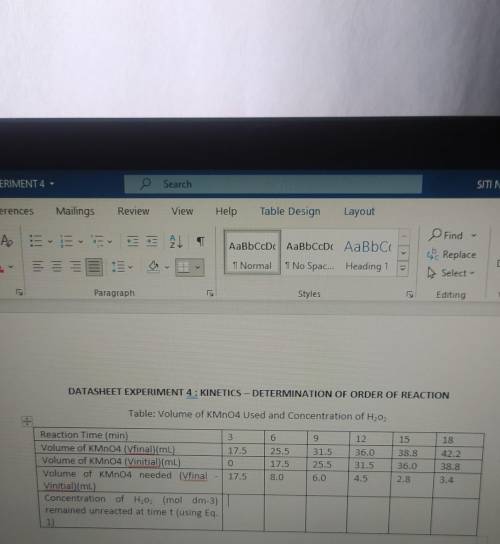
How to find concentration of h2o2
Using eq 1 which is
5HO₂ + 2MnO (4-) +6H+ → 2Mn²+ + 8H₂O +...

Chemistry, 20.06.2021 14:00 fireman59937
How to find concentration of h2o2
Using eq 1 which is
5HO₂ + 2MnO (4-) +6H+ → 2Mn²+ + 8H₂O + 50₂
Given
a:2, b:5
a=kmno4, b=h202
Formula is
MaVa/ MbVb = a/b
a b is coefficient

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:30
Reaction rate depends on how many molecules are coming into contact with each other with enough energy to react. increasing the temperature of the reactants will increase -
Answers: 3

Chemistry, 22.06.2019 18:00
Mercury turns to vapor at 629.88 k how much heat is lost 175 g of mercury vapor at 650 current condenses to a liquid at 297 ca mercury turns to weber at 629.88 kelvin how much he is lost 175 g of mercury vapor and 650 coming condensers to liquidate 297 kevin
Answers: 2

Chemistry, 22.06.2019 20:30
Which states of matter have particles that move independently of one another with very little attraction?
Answers: 1

You know the right answer?
Questions




Mathematics, 20.04.2021 16:30

Advanced Placement (AP), 20.04.2021 16:30

Mathematics, 20.04.2021 16:30

Social Studies, 20.04.2021 16:30


Physics, 20.04.2021 16:30

Mathematics, 20.04.2021 16:30



Mathematics, 20.04.2021 16:30



Computers and Technology, 20.04.2021 16:30

Mathematics, 20.04.2021 16:30

Physics, 20.04.2021 16:30


Mathematics, 20.04.2021 16:30



