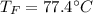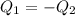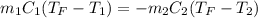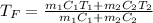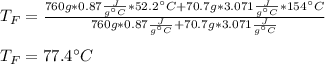Two objects are brought into contact Object 1 has mass 0.76 kg, specific heat capacity 0.87) g'c and initial temperature 52.2 'C. Object #2 has mass 70.7 9. specific heat capacity 3.071" "c' and initial temperature 154 *C. What is the final temperature of the two masses after thermal equilibrium has been reached? Assume the two objects are thermally isolated from everything else. Express your answer in C

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:30
It takes 945.kj/mol to break a nitrogen-nitrogen triple bond. calculate the maximum wavelength of light for which a nitrogen-nitrogen triple bond could be broken by absorbing a single photon.
Answers: 3

Chemistry, 21.06.2019 22:00
Describe the particles of all three phases of matter in the root beer float. (how are the particles arranged and moving? )
Answers: 3

Chemistry, 22.06.2019 07:00
What is the main purpose of patent attorneys? defend the company against legal claims manage financial investments invent new products protect rights to new products and processes
Answers: 1

Chemistry, 22.06.2019 09:30
Based on its chemical properties, identify the position of each chemical family on the periodic table.
Answers: 3
You know the right answer?
Two objects are brought into contact Object 1 has mass 0.76 kg, specific heat capacity 0.87) g'c and...
Questions

Mathematics, 28.01.2021 22:50

History, 28.01.2021 22:50


Mathematics, 28.01.2021 22:50


Advanced Placement (AP), 28.01.2021 22:50


Spanish, 28.01.2021 22:50

Mathematics, 28.01.2021 22:50


Social Studies, 28.01.2021 22:50

History, 28.01.2021 22:50

English, 28.01.2021 22:50

English, 28.01.2021 22:50

Mathematics, 28.01.2021 22:50

Mathematics, 28.01.2021 22:50

Biology, 28.01.2021 22:50

Mathematics, 28.01.2021 22:50