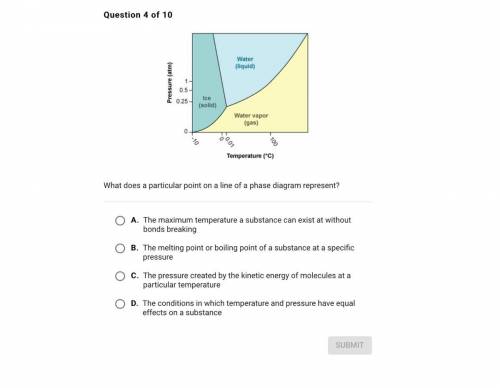
Question 4 of 10
Water
(liquid)
Pressure (atm)
1
0.5-
0.25
Ice<...

Question 4 of 10
Water
(liquid)
Pressure (atm)
1
0.5-
0.25
Ice
(solid)
Water vapor
(gas)
0
160.01
-10
100
Temperature (°C)
What does a particular point on a line of a phase diagram represent?
A. The maximum temperature a substance can exist at without
bonds breaking
B. The melting point or boiling point of a substance at a specific
pressure
C. The pressure created by the kinetic energy of molecules at a
particular temperature
D. The conditions in which temperature and pressure have equal
effects on a substance

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:30
Each pair of clay balls represents to planetesimals if each plane test molluscum pound of the same material and is separated by the same distance which pair experiences the greatest gravitational attraction
Answers: 2

Chemistry, 22.06.2019 13:30
Astudent is trying to create a table that compares hypotheses, theories, and laws. hypothesis theory law do scientific researchers formulate it? yes yes yes does it explain why things happen? yes yes no yes yes yes is it used to make predictions? no yes yes which of the following questions would most likely fill the blank in the table? is it an intelligent guess? is it newly formulated? is it based on observations? has it been proved?
Answers: 1

Chemistry, 22.06.2019 21:00
Use the measurements in the table to determine which unidentified metal has the highest density. metal volume mass a 10.5 cm3 122 g b 14.2 cm3 132 g c 16.1 cm3 115 g d 12.7 cm3 126 g
Answers: 2

You know the right answer?
Questions

Mathematics, 06.04.2020 06:51

Mathematics, 06.04.2020 06:51

English, 06.04.2020 06:51


Mathematics, 06.04.2020 06:51

Engineering, 06.04.2020 06:51

Biology, 06.04.2020 06:51

Health, 06.04.2020 06:51



Mathematics, 06.04.2020 06:52


Mathematics, 06.04.2020 06:52

Mathematics, 06.04.2020 06:52



Biology, 06.04.2020 06:52

English, 06.04.2020 06:52


Mathematics, 06.04.2020 06:52



