Chemistry, 21.06.2021 15:50 deaishaajennings123
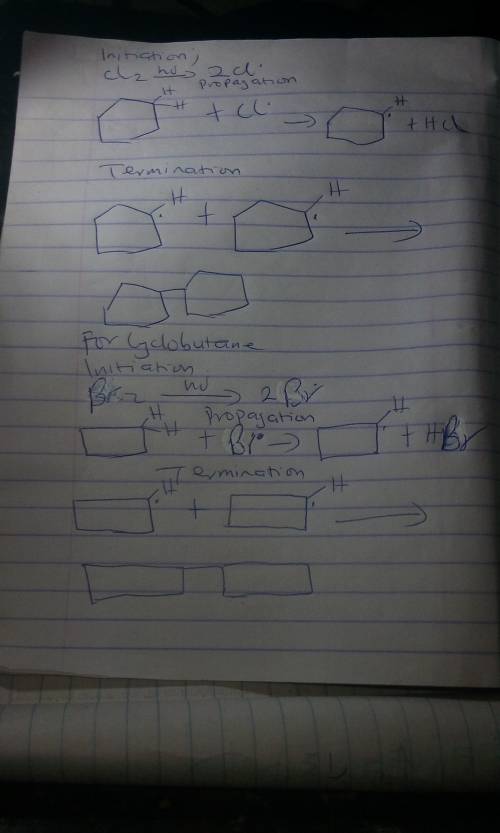
Write the step-by-step mechanism for the following reactions showing, initiation, propogation and atleast one termination rections.
a. The light-initiated reaction of cyclohexane with chlorine to give chlorocyclohexane
b. The reaction between cyclobutane and bromine to give bromocyclobutane

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 16:00
Based on the law of conservation of energy, which statement is false? answer- energy is lost when machines dont work right
Answers: 1

Chemistry, 21.06.2019 22:00
During chemistry class, carl performed several lab tests on two white solids. the results of three tests are seen in the data table. based on this data, carl has concluded that substance b must have bonds.
Answers: 2

Chemistry, 22.06.2019 09:20
Explain that newton first law,second law and third law of motion?
Answers: 2

Chemistry, 22.06.2019 13:00
Lab reagent, hypothesis test.a reference solution used as a lab reagent is purported to have a concentration of 5 mg/dl. six samples are taken from this solution and the following concentrations are recorded: (5.32, 4.88, 5.10, 4.73, 5.15, 4.75) mg/dl.these six measurements are assumed to be an srs of all possible measurements from solution.they are also assumed to have a standard deviation of 0.2, a normal distributin, and a mean concentration equal to the true concentration of the solution.carry out a significance test to determine whether these six measurements provide reliable evidence that the true concentration of the solution is actually not 5 mg/dl.
Answers: 1
You know the right answer?
Write the step-by-step mechanism for the following reactions showing, initiation, propogation and at...
Questions


Mathematics, 27.12.2019 19:31





Mathematics, 27.12.2019 19:31

Advanced Placement (AP), 27.12.2019 19:31


Advanced Placement (AP), 27.12.2019 19:31


Mathematics, 27.12.2019 19:31

Social Studies, 27.12.2019 19:31

Mathematics, 27.12.2019 19:31

History, 27.12.2019 19:31


Mathematics, 27.12.2019 19:31

History, 27.12.2019 19:31


History, 27.12.2019 19:31