gas

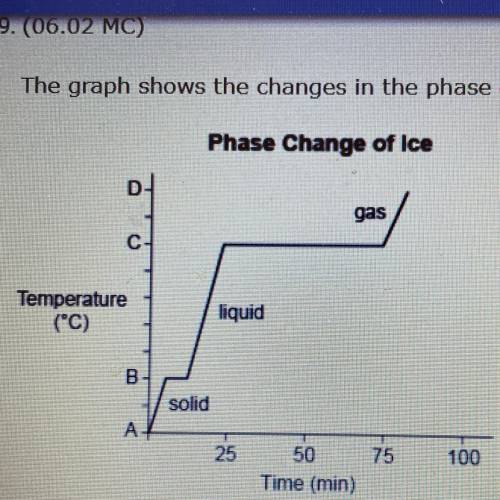
The graph shows the changes in the phase of ice when it is heated.
Phase Change of Ice
gas
C
Temperature
(C)
liquid
в.
solid
A-
25
50
75
100
Time (min)
Which of the following temperatures describes the value of A? (5 points)
0 °C, because it is the melting point gf ice.
O 0 °C, because it is the freezing point of water.
Less than 0 °C, because B represents the temperature at which ice melts.
Less than 0 °C, because B represents the temperature at which water evaporates.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:30
Right anwser gets marked brainliest newton's discovery concerning how fast an object will change speed is the: 1st law 2nd law 3rd law universal gravitation
Answers: 1

Chemistry, 22.06.2019 12:30
If anyone would be able to me out with these three questions it would be these are from the chem 2202 course.
Answers: 3

Chemistry, 22.06.2019 16:40
Let the ed50 of a recreational drug be defined as the amount required for 50% of a test group to feel high or get a buzz. if the ed50 value of ethanol is 470 mg/kg body mass, what dose would a 70 kg party goer need to quickly consume in order to have a 50% chance of getting a buzz? 235 mg 470 mg 32,900 mg 35,000,000 mg
Answers: 3

Chemistry, 22.06.2019 18:00
Hydrogenation reactions, in which h2 and an "unsaturated" organic compound combine, are used in the food, fuel, and polymer industries. in the simplest case, ethene (c2h4) and h2 form ethane (c2h6). if 140 kj is given off per mole of c2h4 reacting, how much heat (in mj) is released when 12 kg of c2h6 forms?
Answers: 2
You know the right answer?
The graph shows the changes in the phase of ice when it is heated.
Phase Change of Ice
gas
gas
Questions


History, 17.11.2019 07:31

Biology, 17.11.2019 07:31

English, 17.11.2019 07:31


Spanish, 17.11.2019 07:31



Mathematics, 17.11.2019 07:31


Mathematics, 17.11.2019 07:31

Biology, 17.11.2019 07:31



History, 17.11.2019 07:31

History, 17.11.2019 07:31


Mathematics, 17.11.2019 07:31

English, 17.11.2019 07:31



