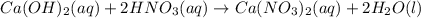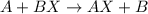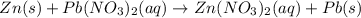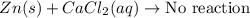Chemistry, 22.06.2021 06:10 chenepiernas
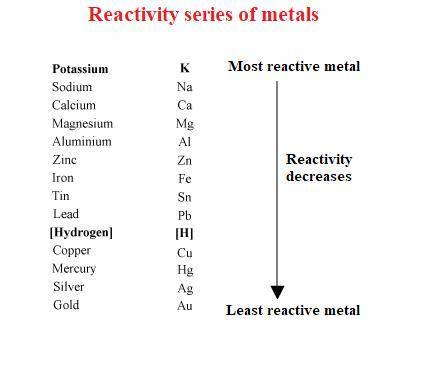
Part 1: Name the type of chemical reaction that occurs when calcium hydroxide (Ca(OH)2) reacts with nitric acid (HNO3). Part 2: Explain why zinc (Zn) would react with lead nitrate (Pb(NO3)2) but not with calcium chloride (CaCl2).

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 23:50
Be sure to answer all parts. the following equilibrium constants were determined at 1123 k: c(s) + co2(g) ⇌ 2co(g) k'p = 1.30 × 1014 co(g) + cl2(g) ⇌ cocl2(g) k''p = 6.00 × 10−3 calculate the equilibrium constant at 1123 k for the reaction: c(s) + co2(g) + 2cl2(g) ⇌ 2cocl2(g) 4.68 × 10 9 (enter your answer in scientific notation.) write the equilibrium constant expression, kp:
Answers: 3

Chemistry, 23.06.2019 05:00
How is electrolysis most commonly used to produce an energy source? a - splitting water molecules produces oxygen, which organisms breathe to fuel their bodies. b - splitting water molecules produces hydrogen gas, which is used to power machines through hydrogen fuel cells. c - splitting carbon dioxide molecules produces coal, a form of carbon that can be burned to produce heat. d - splitting carbon dioxide molecules produces natural gas, which can be burned to generate electricity in power plants.
Answers: 1


Chemistry, 23.06.2019 14:20
Identificaa 5 características que comparten las guacamayas y dos caracteristicas que las hagan diferentes
Answers: 1
You know the right answer?
Part 1: Name the type of chemical reaction that occurs when calcium hydroxide (Ca(OH)2) reacts with...
Questions


History, 14.04.2020 21:22




Mathematics, 14.04.2020 21:22

Biology, 14.04.2020 21:22

Chemistry, 14.04.2020 21:22


History, 14.04.2020 21:22






Chemistry, 14.04.2020 21:23


History, 14.04.2020 21:23


Social Studies, 14.04.2020 21:23