
Chemistry, 22.06.2021 17:10 lilyrockstarmag
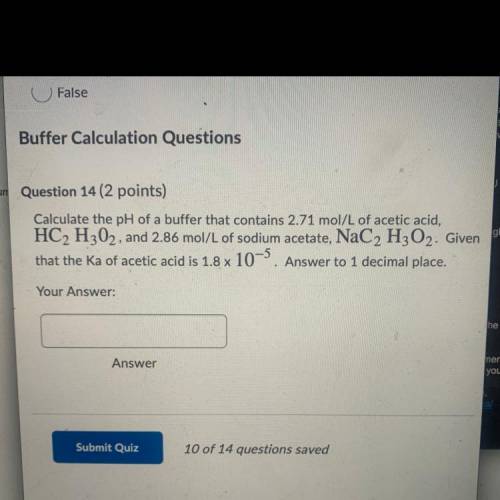
Buffer Calculation Questions
um Question 14 ( points)
Calculate the pH of a buffer that contains 2.71 mol/L of acetic acid,
HC2 H302, and 2.86 mol/L of sodium acetate, NaC2 H2O2. Given
that the Ka of acetic acid is 1.8 x 10-5. Answer to 1 decimal place.
Your
Answer

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:40
What are the resulting coefficients when you balance the chemical equation for the combustion of ethane, c2h6? in this reaction, ethane is burned in the presence of oxygen (o2) to form carbon dioxide (co2) and water (h2o). (g)+(g)→(g)+(g)
Answers: 1

Chemistry, 21.06.2019 19:30
Motivation cannot be developed with practice; a person either possesses it or they do not.
Answers: 1

Chemistry, 22.06.2019 17:00
Reduction is a reaction which results in a in electrons and a in positive charge of the atom or ion 1) a- loss 1) b- gain 2) a-increase 2) b-decrease
Answers: 1

Chemistry, 23.06.2019 01:30
Will a solution form when the solvent and solute are both nonpolar? a. not likely b. never c. most likely
Answers: 1
You know the right answer?
Buffer Calculation Questions
um Question 14 ( points)
Calculate the pH of a buffer that conta...
Calculate the pH of a buffer that conta...
Questions



History, 09.12.2021 22:20




Mathematics, 09.12.2021 22:20

Social Studies, 09.12.2021 22:20

Mathematics, 09.12.2021 22:20


Mathematics, 09.12.2021 22:20

History, 09.12.2021 22:20


Mathematics, 09.12.2021 22:20

History, 09.12.2021 22:20

Mathematics, 09.12.2021 22:20






