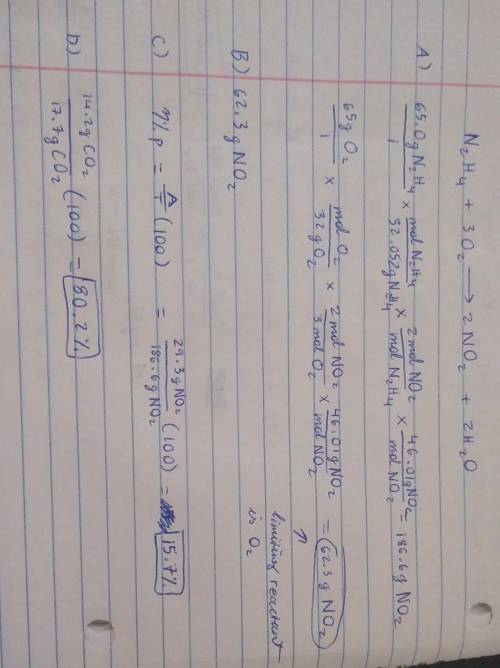19) Hydrazine, N2H4, a substance used as rocket fuel, reacts with oxygen as follows:
N2H4 (1) + 3 O2(g) → 2 NO2(g) + 2 H20 (8)
A) If 65.0 g of hydrazine are reacted with 65.0 g of oxygen, which is the limiting reactant?
usg of Oz X Imol
.
B) How many grams of NO2 are produced from the limiting reactant in part A?
C) If 29.3 g of NO2 are obtained from the reaction in Part A, what is the percent yield?
20) What is the percent yield if 14.2 g of CO2 were produced and 17.7 g were calculated to be
produced from the reaction?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 08:00
Joe shines white light into a bowl half full of water at an angle of incident of 27.5°. calculate the angle of refraction in the water given the indices of refraction for air and water are 1.00 and 1.36, respectively.
Answers: 2


Chemistry, 23.06.2019 01:00
Which statement best describes isomers? a. isomers are alcohols that have the same functional group. b. isomers have at least one carbon-carbon double bond. c. isomers have the same molecular formula but different structural properties.
Answers: 1

Chemistry, 23.06.2019 10:00
Which element forms a compound with chlorine with the general formula mci?
Answers: 1
You know the right answer?
19) Hydrazine, N2H4, a substance used as rocket fuel, reacts with oxygen as follows:
N2H4 (1) + 3 O...
Questions

History, 20.09.2019 06:00



Mathematics, 20.09.2019 06:00




History, 20.09.2019 06:00


History, 20.09.2019 06:00

Mathematics, 20.09.2019 06:00

English, 20.09.2019 06:00



Mathematics, 20.09.2019 06:00


English, 20.09.2019 06:00