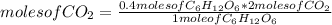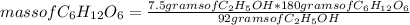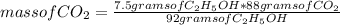Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 21:10
Identify one disadvantage to each of the following models of electron configuration: dot structures arrow and line diagrams written electron configurations type in your answer below.
Answers: 1


Chemistry, 22.06.2019 07:20
Describing intermolecular forces use the drop down menus to match the type of intermolecular force to its name dipole dipole interactions dipole induced dipole interactions london dispersion forces hydrogen bond van der waals forces done
Answers: 1

Chemistry, 22.06.2019 15:00
What is the most important factor in determining climates.
Answers: 1
You know the right answer?
C6H12O6 (aq) -> 2 C2H5OH (aq) + 2 CO2 (g)
a. How many moles of CO2 are produced when 0.400 mol o...
Questions

History, 17.05.2021 23:40

History, 17.05.2021 23:40

History, 17.05.2021 23:40



Mathematics, 17.05.2021 23:40

Mathematics, 17.05.2021 23:40

Mathematics, 17.05.2021 23:40

Chemistry, 17.05.2021 23:40



Mathematics, 17.05.2021 23:40


Mathematics, 17.05.2021 23:40

Mathematics, 17.05.2021 23:40

History, 17.05.2021 23:40



Physics, 17.05.2021 23:40

Mathematics, 17.05.2021 23:40