

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
If 10.g of agno3 is available, what volume of 0.25 m agno3 can be prepared
Answers: 1


Chemistry, 22.06.2019 07:20
2pos suppose an object in free fall is dropped from a building. its starting velocity is 0 m/s. ignoring the effects of air resistance, what is the speed (in m/s) of the object after falling 3 seconds? give your answer as a positive decimal without units. answer here
Answers: 1

Chemistry, 22.06.2019 10:00
How many mmols of tris-hcl are there in 100 ml of a 100 mm tris-hcl buffer solution at ph 8.1? note that the 100 mm refers to the sum of tris and tris-hcl concentrations?
Answers: 3
You know the right answer?
Consider the following intermediate chemical equations.
CH2(g) →C(s) + 2H2(g)
CC1.(g) → C(s)...
CC1.(g) → C(s)...
Questions



Chemistry, 14.01.2021 02:00




Mathematics, 14.01.2021 02:00


Mathematics, 14.01.2021 02:00



Mathematics, 14.01.2021 02:00

Chemistry, 14.01.2021 02:00

English, 14.01.2021 02:00


Computers and Technology, 14.01.2021 02:00

History, 14.01.2021 02:00



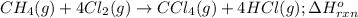
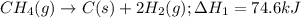
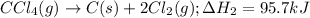
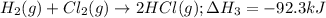
![\Delta H^o_{rxn}=[1\times (\Delta H_1)] + [1\times (-\Delta H_2)] + [2\times (\Delta H_3)]](/tpl/images/1382/1138/89421.png)
![\Delta H^o_{rxn}=[1\times (74.6)] + [1\times (-95.7)] + [2\times (-92.3)]\\\\\Delta H^o_{rxn}=-205.7kJ](/tpl/images/1382/1138/33de2.png)


