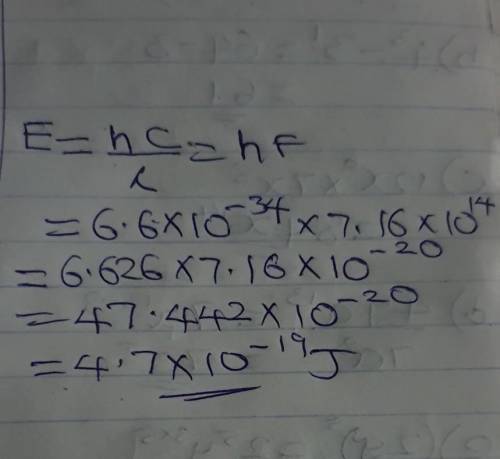Chemistry, 23.06.2021 19:00 biaxialpower789
A certain shade of blue has a frequency of 7.16x10^14Hz. What is the energy of exactly one photon of this light? Planck’s constant h=6.626x10^-34 j•s.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:30
This element exists in adundance in the sun.explain how you would go about capturing sunlight.would this captured sunlight contain any of the element?
Answers: 1

Chemistry, 22.06.2019 03:30
In saturated organic compounds, all the bonds between carbon atoms are called?
Answers: 1

Chemistry, 22.06.2019 12:00
Consider the following reaction at equilibrium. 2co2 (g) 2co (g) + o2 (g) h° = -514 kj le châtelier's principle predicts that the equilibrium partial pressure of co (g) can be maximized by carrying out the reaction a. at high temperature and high pressure b. at high temperature and low pressure c. at low temperature and low pressure d. at low temperature and high pressure e. in the presence of solid carbon
Answers: 2

Chemistry, 22.06.2019 13:30
Which of the following has wavelength longer than the wavelength of viable light? a) x rays b) gamma rays c) radios waves d) ultraviolet waves
Answers: 1
You know the right answer?
A certain shade of blue has a frequency of 7.16x10^14Hz. What is the energy of exactly one photon of...
Questions

Mathematics, 05.10.2021 05:20

Mathematics, 05.10.2021 05:20

Mathematics, 05.10.2021 05:20



Mathematics, 05.10.2021 05:20

Physics, 05.10.2021 05:20


English, 05.10.2021 05:20

SAT, 05.10.2021 05:20

History, 05.10.2021 05:20

SAT, 05.10.2021 05:30

Mathematics, 05.10.2021 05:30


Mathematics, 05.10.2021 05:30

Mathematics, 05.10.2021 05:30

English, 05.10.2021 05:30


Mathematics, 05.10.2021 05:30