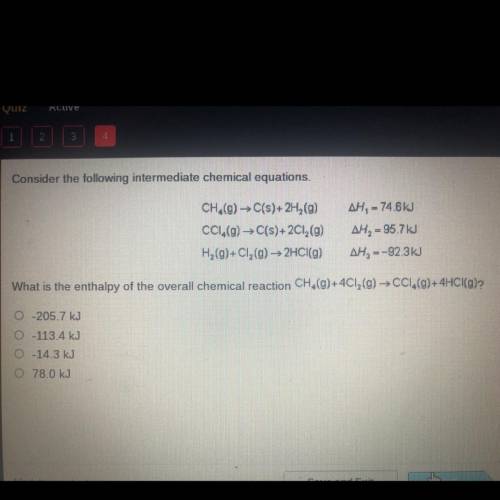
Consider the following intermediate chemical equations.
CH.(g) → C(s)+2H (9)
CC1.(g) → C(s)+2...

Chemistry, 23.06.2021 19:50 nataliajaquez02
Consider the following intermediate chemical equations.
CH.(g) → C(s)+2H (9)
CC1.(g) → C(s)+2Cl2(g)
H2(g)+C1, (g) → 2HCl(g)
AH, = 74.6 kJ
AH, = 95.7 kJ
AH, =-92.3kJ
What is the enthalpy of the overall chemical reaction CH,(g)+4C12(g) → CC1,(9)+4HCI(g)?
O-205.7 kJ
0-113.4 kJ
-14.3 kJ
0 78.0 kJ

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:30
Explain why pure hydrogen cyanide does not conduct electricity, but become a conductor when it is dissolved in water? (at room temp, pure hcn exists as a volatile liquid)
Answers: 1

Chemistry, 22.06.2019 09:40
Apiece of copper has a temperature of 75.6 0c. when the metal is placed in 100.0 grams of water at 19.1 0c, the temperature rises by 5.5 0c. what is the mass of the metal?
Answers: 1

Chemistry, 22.06.2019 11:50
If oil spills continue, all of the following should be expected except (2 points) death of aquatic life. polluted groundwater. decreased soil productivity. increased global temperatures.
Answers: 3

Chemistry, 22.06.2019 12:00
the mississippians were considered to be horticulturalists, which means they were
Answers: 1
You know the right answer?
Questions


Mathematics, 08.12.2020 01:00


Mathematics, 08.12.2020 01:00











Mathematics, 08.12.2020 01:00

Mathematics, 08.12.2020 01:00


Mathematics, 08.12.2020 01:00

Mathematics, 08.12.2020 01:00



