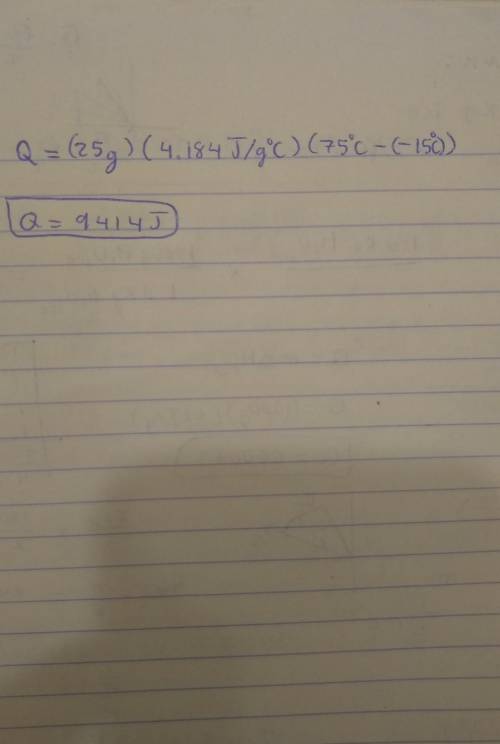Chemistry, 24.06.2021 03:30 sevaramirabell
Note: Please show all work and calculation setups to get full credit. T. he following may be used on this assignment: specific heat of (water=4.184 J/g oC; ice=2.03 J/g oC; steam=1.99 184 J/g oC); heat of fusion of water=80. cal/g; heat of vaporization=540 cal/g; 1cal=4.184J. Calculate the energy required (in J) to convert 25 g of ice at -15 oC to water at 75 oC.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:20
6. what does the symbol ah stand for? o one calorie given off by a reaction the specific heat of a substance the heat capacity of a substance the heat of reaction for a chemical reaction
Answers: 1


Chemistry, 22.06.2019 08:40
Which statement can best be concluded from the ideal gas law?
Answers: 2

Chemistry, 23.06.2019 00:30
Which of the following best describes technology a. something created for only scientists to use b.the method of thinking that scientists use. c.the application of engineering to create useful products. c. a scientific idea
Answers: 1
You know the right answer?
Note: Please show all work and calculation setups to get full credit. T. he following may be used on...
Questions

Health, 29.10.2020 17:30

Physics, 29.10.2020 17:30




Arts, 29.10.2020 17:30

Mathematics, 29.10.2020 17:30


History, 29.10.2020 17:30

English, 29.10.2020 17:30


Chemistry, 29.10.2020 17:30

Mathematics, 29.10.2020 17:30

Mathematics, 29.10.2020 17:30


Chemistry, 29.10.2020 17:30


English, 29.10.2020 17:30