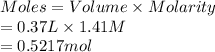Chemistry, 24.06.2021 15:50 brooklynpage5283
A chemist adds 370.0mL of a 1.41/molL potassium iodide KI solution to a reaction flask. Calculate the millimoles of potassium iodide the chemist has added to the flask. Be sure your answer has the correct number of significant digits.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:30
If 3.00 g of titanium metal is reacted with 6.00 g of chlorine gas, cl2, to form 7.7 g of titanium (iv) chloride in a combination reaction, what is the percent yield of the product?
Answers: 1

Chemistry, 22.06.2019 09:40
In the lab, ammonia was mixed with water to form ammonium hydroxide. what is/are the reactant(s)? o water and ammonia o ammonia o ammonium hydroxide need
Answers: 2


You know the right answer?
A chemist adds 370.0mL of a 1.41/molL potassium iodide KI solution to a reaction flask. Calculate th...
Questions


Physics, 26.09.2019 20:30


Biology, 26.09.2019 20:30




Mathematics, 26.09.2019 20:30


Social Studies, 26.09.2019 20:30

Mathematics, 26.09.2019 20:30







Mathematics, 26.09.2019 20:30


Physics, 26.09.2019 20:30