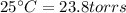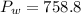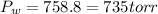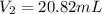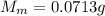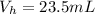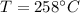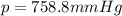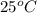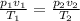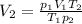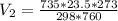4. A piece of metal weighing 0.0713 g was placed in a eudiometer containing dilute aqueous HCl. After the metal fully dissolved, 23.5 mL of hydrogen gas was collected by displace-ment of water and a 400 mm column of water was observed. The water temperature was 258C and the barometric pressure was 758.8 mm Hg (torr). Refer to the Introduction and data sheet to solve the following problems. a) What is the vapor pressure of the water vapor in the column? (Consult Appendix E.)

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
How much heat is released upon converting one mole of steam (18.0 g) from 100.0 ∘c to water at 25.0 ∘c? show work and constants, trying to figure out how it works. only given the heat capacity for steam and water so try to only use that
Answers: 1

Chemistry, 22.06.2019 01:30
Reaction rate depends on how many molecules are coming into contact with each other with enough energy to react. increasing the temperature of the reactants will increase -
Answers: 3

Chemistry, 22.06.2019 09:30
The chart shows the bid provided by four contractors to complete a job. which contractor is the most cost-effective?
Answers: 3

Chemistry, 22.06.2019 19:00
Nan element’s square on the periodic table, the number with the greatest numerical value represents the
Answers: 3
You know the right answer?
4. A piece of metal weighing 0.0713 g was placed in a eudiometer containing dilute aqueous HCl. Afte...
Questions

Spanish, 12.10.2019 00:30


Arts, 12.10.2019 00:30

Mathematics, 12.10.2019 00:30

Physics, 12.10.2019 00:30

Chemistry, 12.10.2019 00:30

Mathematics, 12.10.2019 00:30

Chemistry, 12.10.2019 00:30

Mathematics, 12.10.2019 00:30



Mathematics, 12.10.2019 00:30


English, 12.10.2019 00:40



Mathematics, 12.10.2019 00:40


Mathematics, 12.10.2019 00:40