
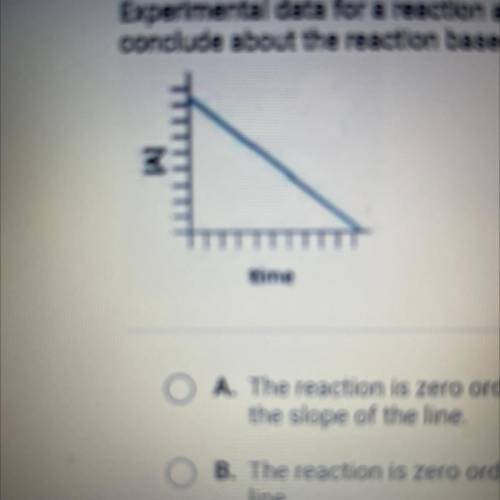
Experimental data for a reaction are collected and graphed. What can you
conclude about the reaction based on the graph?
A. The reaction is zero order, and the rate constant is the negative of
the slope of the line
B. The reaction is zero order, and the rate constant is the slope of the
line
C. The reaction is first order, and the rate constant is the slope of the
line,
D. The reaction is first order, and the rate constant is the negative eg

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:20
6. what does the symbol ah stand for? o one calorie given off by a reaction the specific heat of a substance the heat capacity of a substance the heat of reaction for a chemical reaction
Answers: 1

Chemistry, 22.06.2019 09:20
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 19:50
If a gas has an initial pressure of 101kpa and a volume of 10l, then it expands to a volume of 20l, what is the new pressure?
Answers: 2

Chemistry, 22.06.2019 22:00
Plz ill give u brainliest which of the following steps is not likely to take place during cellular respiration? a.oxygen combines with carbon of simple sugar. b. energy molecule transfers energy to cells. d. energy is used up.
Answers: 3
You know the right answer?
Experimental data for a reaction are collected and graphed. What can you
conclude about the reactio...
Questions

Mathematics, 09.11.2019 02:31


Mathematics, 09.11.2019 02:31

Mathematics, 09.11.2019 02:31


Mathematics, 09.11.2019 02:31

Mathematics, 09.11.2019 02:31


Mathematics, 09.11.2019 02:31

Health, 09.11.2019 02:31


History, 09.11.2019 02:31

English, 09.11.2019 02:31

Mathematics, 09.11.2019 02:31


Mathematics, 09.11.2019 02:31




Chemistry, 09.11.2019 02:31



