
Chemistry, 24.06.2021 20:10 emmarieasimon
A certain liquid has a normal freezing point of and a freezing point depression constant . Calculate the freezing point of a solution made of of iron(III) chloride () dissolved in of . Round your answer to significant digits.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 11:30
Voltaic cells produce a positive overall charge. what does this indicate? a. the reaction is likely to be endothermic. b. the reaction is spontaneous. c. the reaction is not likely to occur. d. the reaction is not spontaneous.
Answers: 3

Chemistry, 22.06.2019 15:00
20 pts ‼️ an unmanned spacecraft travels to mars. mars has a lower strength of gravity than earth. where in the image is the spacecraft’s weight the greatest?
Answers: 1

Chemistry, 22.06.2019 23:00
Which of your 24 wells had indications that a chemical reaction occurred? how were you able to tell that a chemical reaction occurred? which of your 24 wells had indications that a physical reaction occurred? how were you able to tell that a physical reaction occurred? report on both mixing and evaporation. make a general statement about whether your hypotheses were validated or rejected. must your hypotheses be correct for this to be a successful laboratory?
Answers: 3

Chemistry, 23.06.2019 00:00
In an exothermic reaction, energy may be released to the surroundings in the form of question 4 options: heat light thermal all of the above
Answers: 3
You know the right answer?
A certain liquid has a normal freezing point of and a freezing point depression constant . Calculate...
Questions



English, 27.04.2020 01:21






Physics, 27.04.2020 01:21

Mathematics, 27.04.2020 01:21

Mathematics, 27.04.2020 01:21


Spanish, 27.04.2020 01:21

Chemistry, 27.04.2020 01:21


Biology, 27.04.2020 01:21


Spanish, 27.04.2020 01:21

Mathematics, 27.04.2020 01:21

Mathematics, 27.04.2020 01:21

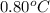 and a freezing point depression constant
and a freezing point depression constant 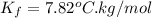 . Calculate the freezing point of a solution made of 81.1 g of iron(III) chloride () dissolved in 850. g of X. Round your answer to significant digits.
. Calculate the freezing point of a solution made of 81.1 g of iron(III) chloride () dissolved in 850. g of X. Round your answer to significant digits.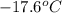
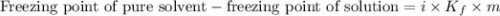
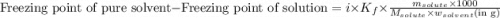 ......(1)
......(1)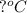
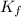 = freezing point depression constant =
= freezing point depression constant = 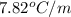
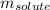 = Given mass of solute (iron (III) chloride) = 81.1 g
= Given mass of solute (iron (III) chloride) = 81.1 g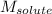 = Molar mass of solute (iron (III) chloride) = 162.2 g/mol
= Molar mass of solute (iron (III) chloride) = 162.2 g/mol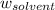 = Mass of solvent (X) = 850. g
= Mass of solvent (X) = 850. g![0.8-(\text{Freezing point of solution})=4\times 7.82\times \frac{81.1\times 1000}{162.2\times 850}\\\\\text{Freezing point of solution}=[0.8-18.4]^oC\\\\\text{Freezing point of solution}=-17.6^oC](/tpl/images/1383/4070/c99f0.png)


