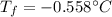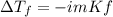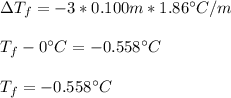Chemistry, 24.06.2021 20:20 daebreonnakelly
Calculate the freezing point of a 0.100 m aqueous solution of K2SO4, taking interionic attractions into consideration by using the van't Hoff factor (i for 0.100 m K2SO4

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:00
What is the main purpose of patent attorneys? defend the company against legal claims manage financial investments invent new products protect rights to new products and processes
Answers: 1

Chemistry, 22.06.2019 08:30
The mass of a neutron is equal to the mass of a proton plus the mass of an electron. true or false false true
Answers: 1

Chemistry, 22.06.2019 14:00
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3

Chemistry, 22.06.2019 17:10
Increasing the substrate concentration in an enzymatic reaction could overcome which of the following? a) the need for a coenzymeb) allosteric inhibitionc) competitive inhibitiond) insufficient cofactors
Answers: 1
You know the right answer?
Calculate the freezing point of a 0.100 m aqueous solution of K2SO4, taking interionic attractions i...
Questions

Biology, 22.07.2019 15:30


Chemistry, 22.07.2019 15:30

History, 22.07.2019 15:30


Biology, 22.07.2019 15:30

Mathematics, 22.07.2019 15:30

History, 22.07.2019 15:30

Biology, 22.07.2019 15:30

English, 22.07.2019 15:30

Physics, 22.07.2019 15:30


Biology, 22.07.2019 15:30

Computers and Technology, 22.07.2019 15:30


History, 22.07.2019 15:30