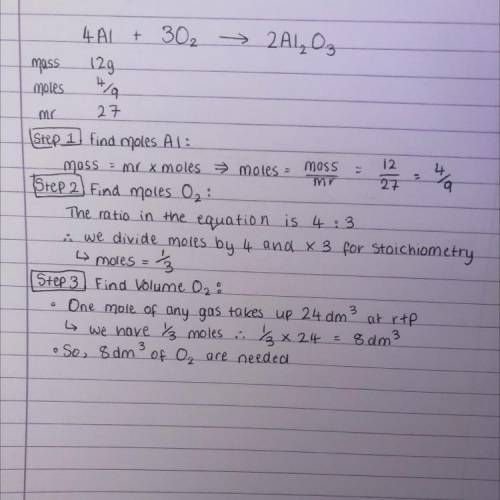Chemistry, 24.06.2021 20:50 lollipop8011
4. Aluminium combines with oxygen to form aluminium oxide via the equation below. When 12g of aluminium is reacted, calculate the volume of O2 that is needed at rtp. 4Al + 3O2 > 2Al2O3

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:00
How many moles are in 7.2 x 10^23 carbon molecules? (*round to the nearest hundredth and include the unit "mol c" after your number) question 6 options:
Answers: 2


Chemistry, 22.06.2019 19:20
The equation picture below shows which type of nuclear reaction u 235 + n x e 134 + sr 100 + 2n
Answers: 1

Chemistry, 22.06.2019 19:40
What is the wavelength of a 3*10^12 hz infrared wave a 3*10^20m b 1* 10^4m c 3*10^-3m d 1*10^-4 m
Answers: 1
You know the right answer?
4. Aluminium combines with oxygen to form aluminium oxide via the equation below. When 12g of alumin...
Questions

Biology, 10.12.2020 23:20

Arts, 10.12.2020 23:20


Mathematics, 10.12.2020 23:20


English, 10.12.2020 23:20




Mathematics, 10.12.2020 23:20


Mathematics, 10.12.2020 23:20



Social Studies, 10.12.2020 23:20


Mathematics, 10.12.2020 23:20



Mathematics, 10.12.2020 23:20