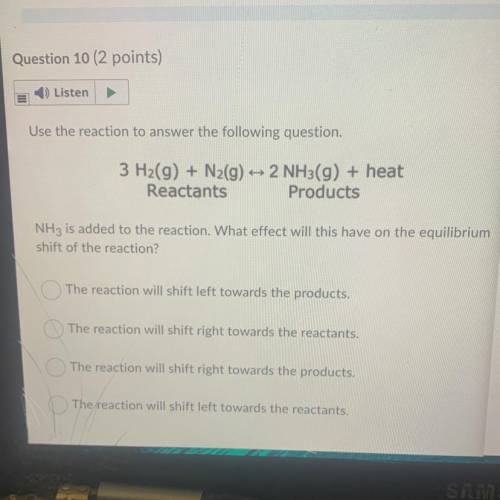
Use the reaction to answer the following question.
3 H2(g) + N2(g) + 2 NH3(g) + heat
Reactant...

Use the reaction to answer the following question.
3 H2(g) + N2(g) + 2 NH3(g) + heat
Reactants Products
NH3 is added to the reaction. What effect will this have on the equilibrium
shift of the reaction?
The reaction will shift left towards the products.
The reaction will shift right towards the reactants.
The reaction will shift right towards the products.
The reaction will shift left towards the reactants.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:00
Match each object to its description: a. coma of a comet b. comet tail c. oort cloud haze surrounding a nucleus created by solar wind. hypothetical sphere around the solar system
Answers: 1

Chemistry, 22.06.2019 06:00
Calculate - analysis of compound composed of iron and oxygen yields 174.86 of fe and 75.14g of o. what is the empirical formula for this compound?
Answers: 3

Chemistry, 22.06.2019 09:00
Scientific evidence tells us that the cause of earths four season is the tilt of earth as it revolves around the sun. the student is instructed to illustrate this information in a science notebook. how will the student illiterate winter in the northern hemisphere?
Answers: 3

Chemistry, 22.06.2019 11:30
Voltaic cells produce a positive overall charge. what does this indicate? a. the reaction is likely to be endothermic. b. the reaction is spontaneous. c. the reaction is not likely to occur. d. the reaction is not spontaneous.
Answers: 3
You know the right answer?
Questions


Physics, 21.07.2019 03:31


Biology, 21.07.2019 03:31

History, 21.07.2019 03:31

Social Studies, 21.07.2019 03:31


Geography, 21.07.2019 03:31

Mathematics, 21.07.2019 03:31

Mathematics, 21.07.2019 03:31

Chemistry, 21.07.2019 03:31

Mathematics, 21.07.2019 03:31

Mathematics, 21.07.2019 03:31

Mathematics, 21.07.2019 03:31




History, 21.07.2019 03:31




