Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:00
Is this a scientific model? use complete sentences to explain why or why not. a graphic organizer showing the water cycle i need : ( asap i go it never mind
Answers: 2

Chemistry, 22.06.2019 03:10
Between 2014 and 2016, more than 25,000 children in flint, michigan, drank water that was contaminated with lead from lead pipes. during this time, the city claimed the water was safe to drink. which of these actions could the city have taken to ensure that the drinking water was free from lead?
Answers: 3

Chemistry, 22.06.2019 06:10
56.16 gregor mendel was the first scientist to use statistics to analyze scientific data. before mendel's experiments, scientists believed that organisms acquired traits from their environment and passed them on to their offspring. after mendel's discoveries were accepted, scientists realized that traits passed to offspring were the result of genes being passed from parents to offspring. this is an example of pls
Answers: 1

Chemistry, 22.06.2019 11:00
What is the molar mass of a gas that has density of 2.054 g/l
Answers: 2
You know the right answer?
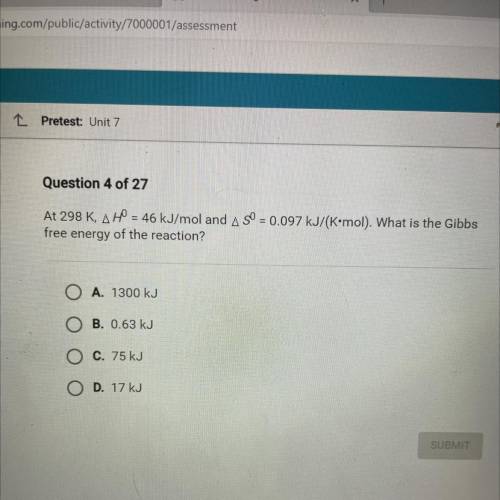
At 298 K, AH = 46 kJ/mol and A SO = 0.097 kJ/(K•mol). What is the Gibbs
free energy of the reaction...
Questions

Mathematics, 04.03.2021 09:40

Physics, 04.03.2021 09:40

Mathematics, 04.03.2021 09:40


Mathematics, 04.03.2021 09:40

Mathematics, 04.03.2021 09:40



Mathematics, 04.03.2021 09:40

Mathematics, 04.03.2021 09:40


Mathematics, 04.03.2021 09:40

Spanish, 04.03.2021 09:40

Mathematics, 04.03.2021 09:40


Mathematics, 04.03.2021 09:40


Mathematics, 04.03.2021 09:40

Mathematics, 04.03.2021 09:40