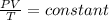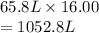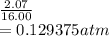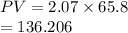Chemistry, 25.06.2021 03:10 Christyhanes3764
A flexible vessel contains 65.8 L of gas at a pressure of 2.07 atm. Under the conditions of constant temperature and constant number of moles of gas, what is the pressure of the gas (in atm) when the volume of the vessel increased by a factor of 16.00

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:30
What is i fracture in the crust called when land move up, down or sideways
Answers: 2

Chemistry, 22.06.2019 13:30
Which statements are true concerning mineral formation? check all that apply. the slower the cooling, the larger the crystals. the faster the cooling, the smaller the crystals. crystals formed from magma are smaller than crystals formed from lava. minerals can only form in solutions when the solution is heated deep underground. when a solution cools, elements and compounds leave the solution and crystallize as minerals. minerals formed from hot water solutions can form narrow channels in the surrounding rock.
Answers: 1

Chemistry, 23.06.2019 02:30
Which statement best describes the liquid state of matter? a. it has definite shape but indefinite volume. b. it has definite shape and definite volume. c. it has indefinite shape and indefinite volume. d. it has indefinite shape but definite volume.
Answers: 1

Chemistry, 23.06.2019 10:10
Calculate the h3o+ concentration in a solution of acetic acid if the concentration of molecular acetic acid present at equilibrium is 9.97x10-3 m and k for the dissociation is 1.86x10-5. ch3cooh(aq)+h2o(l)+> h3o+(aq)+ch3coo-(aq) show me how to get the answer.
Answers: 3
You know the right answer?
A flexible vessel contains 65.8 L of gas at a pressure of 2.07 atm. Under the conditions of constant...
Questions

Mathematics, 10.05.2021 17:40

Mathematics, 10.05.2021 17:40

English, 10.05.2021 17:40

English, 10.05.2021 17:40

Mathematics, 10.05.2021 17:40



Mathematics, 10.05.2021 17:40

Mathematics, 10.05.2021 17:40

English, 10.05.2021 17:40


Mathematics, 10.05.2021 17:40




Mathematics, 10.05.2021 17:40

Mathematics, 10.05.2021 17:40


Mathematics, 10.05.2021 17:40

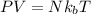
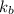 = Boltzmann constant
= Boltzmann constant