
Chemistry, 25.06.2021 05:20 gudon986732
A chemist must prepare 900.0 mL of potassium hydroxide solution with a pH of 12.20 at 25°C.
He will do this in three steps:
• Fill a 900.0 mL volumetric flask about halfway with distilled water.
• Weigh out a small amount of solid potassium hydroxide and add it to the flask.
. Fill the flask to the mark with distilled water.
Calculate the mass of potassium hydroxide that the chemist must weigh out in the second step. Round your answer to 2 significant digits and put your answer in grams (g).

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:30
The reaction q+r2=r2q is found to be first order in r2 and
Answers: 1

Chemistry, 22.06.2019 14:50
Complete the following statements to describe solids, liquids, and gases. select the correct answer from each drop-down menu. a solid a definite volume and a definite shape. a liquid a definite volume and a definite shape. a gas a definite volume and a definite shape
Answers: 1


Chemistry, 23.06.2019 08:40
Which statement is true according to the kinetic theory? a. molecules of different gases with the same mass and temperature always have the same average density. b. molecules of different gases with the same mass and temperature always have the same average volume. c. molecules of different gases with the same mass and temperature always have the same pressure. d. molecules of different gases with the same mass and temperature always have the same molecular mass. e. molecules of different gases with the same mass and temperature always have the same average kinetic energy.
Answers: 1
You know the right answer?
A chemist must prepare 900.0 mL of potassium hydroxide solution with a pH of 12.20 at 25°C.
He will...
Questions


Chemistry, 09.04.2021 14:00


Business, 09.04.2021 14:00




English, 09.04.2021 14:00

Mathematics, 09.04.2021 14:00

Business, 09.04.2021 14:00


Mathematics, 09.04.2021 14:00


Mathematics, 09.04.2021 14:00

English, 09.04.2021 14:00

History, 09.04.2021 14:00



Computers and Technology, 09.04.2021 14:00

Physics, 09.04.2021 14:00

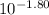 = 0.0158 M
= 0.0158 M

