
Chemistry, 25.06.2021 14:00 maggie9459
A mixture of reactants and products for the reacting shown below is at equilibrium 2.0 L container. What would most likely happen to the equilibrium if the volume of the container were increased to 4.0 L?
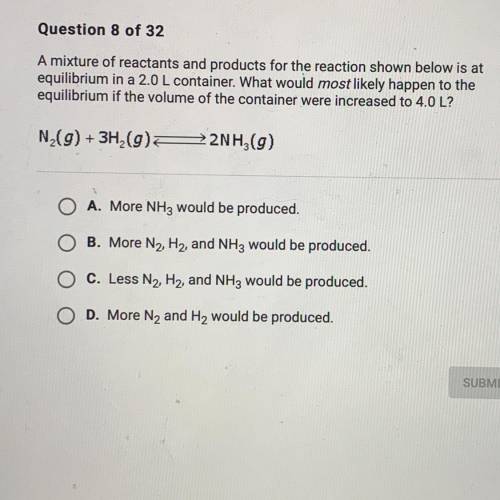

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:20
Use the gizmo to find the concentration of the mystery ch3cooh. use the titrant and indicator shown below perform the titration. what is the titrant volume? titrant analyte indicator titrant volume analyte concentration naoh ch3cooh phenophthalein select one: a. 20.0 ml b. 27.0 ml c. 30.0 ml d. 24.0 ml
Answers: 2

Chemistry, 22.06.2019 07:50
In which situation can a mixture always be called a solution
Answers: 3

Chemistry, 22.06.2019 09:30
Apump contains 0.5 l of air at 203 kpa.you draw back on the piston of the pump, expanding the volume until the pressure reads 50.8 kpa. what is the new volume of the air pump
Answers: 2

You know the right answer?
A mixture of reactants and products for the reacting shown below is at equilibrium 2.0 L container....
Questions


Social Studies, 31.07.2019 22:00


Chemistry, 31.07.2019 22:00





Mathematics, 31.07.2019 22:00


Biology, 31.07.2019 22:00

Mathematics, 31.07.2019 22:00

Mathematics, 31.07.2019 22:00

Business, 31.07.2019 22:00

Mathematics, 31.07.2019 22:00

Advanced Placement (AP), 31.07.2019 22:00

Social Studies, 31.07.2019 22:00


History, 31.07.2019 22:00



