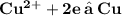Chemistry, 26.06.2021 17:10 annie2u559
Which reaction occurs at the cathode of a galvanic cell that has an aluminum electrode in an electrolyte with aluminum ions and copper electrode in an electrolyte with copper ions ? The reduction potential for the reduction of Cu^ 2+ = 0.34 The reduction potential for the reduction of AP^ 3+ =-1.68V.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:30
98 ! and brainliest plz ! the below reaction can be categorized as more than one type of reaction. which reactions are these, and what are the types of reactions?
Answers: 1

Chemistry, 22.06.2019 09:00
Given the following reaction: c3h8+5o2=3co2+4h20 how many grams of co2 will be produced 7 g of c3h8 and 98 g of o2
Answers: 1

Chemistry, 22.06.2019 10:30
Consider the following reactions. (note: (s) = solid, (l) = liquid, and (g) = gas.) mg(s) + ½o2(g) → mgo(s) + 146 kcal/mole h2(g) + ½o2(g) → h2o(g), δh = -57.82 kcal/mole what type of reaction is represented by the previous two examples?
Answers: 3

Chemistry, 22.06.2019 13:00
16. why must the number of electrons lost equal the number of electrons gained in every redox reaction? use 3 – 4 sentences in your own words to address this question. 18. what type of radiation is emitted when chromium-51 decays into manganese-51? show the nuclear equation that leads you to this answer. 19. a radioactive nucleus alpha decays to yield a sodium-24 nucleus in 14.8 hours. what was the identity of the original nucleus? show the nuclear equation that leads you to this answer.
Answers: 2
You know the right answer?
Which reaction occurs at the cathode of a galvanic cell that has an aluminum electrode in an electro...
Questions


History, 10.02.2021 07:30

Biology, 10.02.2021 07:30

Mathematics, 10.02.2021 07:30

Computers and Technology, 10.02.2021 07:30


Mathematics, 10.02.2021 07:30




Mathematics, 10.02.2021 07:30



Mathematics, 10.02.2021 07:30

Mathematics, 10.02.2021 07:30

Chemistry, 10.02.2021 07:30