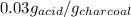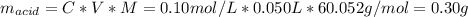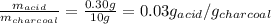Chemistry, 27.06.2021 14:00 raywils0n12300p0t3yc
50 ml of 0.2 M acetic acid is shaken with 10 g activated charcoal. The concentration of acetic acid is reduced to 0.5 times the original molarity. The amount of solute (in g) adsorbed per gram of the adsorbent is approximately

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:10
The enthalpy of formation of water is -285.8 kj/mol. what can be inferred from this statement?
Answers: 1


Chemistry, 22.06.2019 22:30
Which of the following is not an assumption that scientists must make about the natural world? a. regularity b. causality c. predictability d. plausibility
Answers: 1

Chemistry, 22.06.2019 23:00
The data below were determined for the reaction shown below. s2o82– + 3i – (aq) → 2so42– + i3– expt. # [s2o82–] (m) [i –] (m) initial rate 1 0.038 0.060 1.4 × 10 – 5 m/s 2 0.076 0.060 2.8 × 10 – 5 m/s 3 0.076 0.030 1.4 × 10 – 5 m/s the rate law for this reaction must be:
Answers: 1
You know the right answer?
50 ml of 0.2 M acetic acid is shaken with 10 g activated charcoal. The concentration of acetic acid...
Questions

English, 15.04.2021 03:10

Mathematics, 15.04.2021 03:10

Mathematics, 15.04.2021 03:10



Mathematics, 15.04.2021 03:10


French, 15.04.2021 03:10



Mathematics, 15.04.2021 03:10

History, 15.04.2021 03:10



Mathematics, 15.04.2021 03:10

History, 15.04.2021 03:10

Mathematics, 15.04.2021 03:10