Chemistry, 28.06.2021 15:40 negativechill
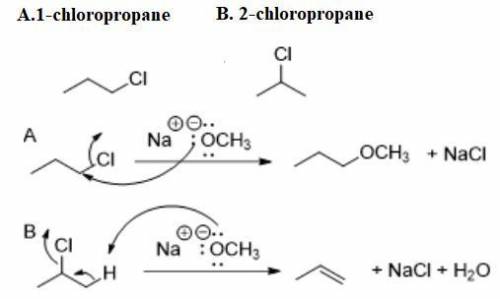
Compound A and compound B are constitutional isomers with molecular formula C3H7Cl. When compound A is treated with sodium methoxide, a substitution reaction predominates. When compound B is treated with sodium methoxide, an elimination reaction predominates.
Required:
Propose structures A and B.

Answers: 1


Another question on Chemistry

Chemistry, 23.06.2019 05:40
Why is it incorrect to balance a chemical equation by changing the subscripts? explain.
Answers: 2

Chemistry, 24.06.2019 03:30
What object exerts the greatest gravitational force within our solar system? explain whether that is the only gravitational force that affects objects in our solar system.
Answers: 2

Chemistry, 24.06.2019 04:00
Methane reacts with oxygen in the following combustion reaction. ch4 + 2o2 → co2 + 2h2o what bonds are broken in this reaction? a. four c–h single bonds and two o=o double bonds b. four c–h single bonds c. one c=o double bond and four o–h single bonds d. two o=o double bonds
Answers: 2

Chemistry, 24.06.2019 07:40
Which example best represents the adhesion, cohesion, and surface tension of water? ice forming on the surface of water bodies in wintersthe ability of water to dissolve many substanceswater droplets forming a bead-like shape over a glass table
Answers: 3
You know the right answer?
Compound A and compound B are constitutional isomers with molecular formula C3H7Cl. When compound A...
Questions









Mathematics, 13.05.2021 22:10

Computers and Technology, 13.05.2021 22:10







Mathematics, 13.05.2021 22:10


Mathematics, 13.05.2021 22:10

History, 13.05.2021 22:10