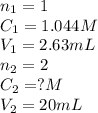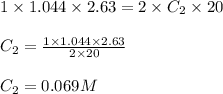Chemistry, 28.06.2021 18:40 summerjoiner
Standardization of a Borax solution (Na2B4O7). You are given a 1.044 M solution of H2SO4. It takes 2.63 mL of this H2SO4 to reach the end point. Knowing it takes 1 H2SO4 to neutralize 2 Na2B4O7, what was the concentration of this Borax solution?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:30
Aroller coaster car is traveling down a track at 22 m/s. the car has a mass of 2000 kg. what is the kinetic energy of the car? a) 22,000 j b) 968,000 j c) 484,000 j d) 44,000 j
Answers: 2

Chemistry, 22.06.2019 01:50
7. what temperature is need to just dissolve 50 g of nh4cl in 75 g of water? '
Answers: 1

Chemistry, 22.06.2019 04:00
What three natural resources are found in the great lakes region
Answers: 2

Chemistry, 22.06.2019 05:30
Compare and contrast physical changes with chemical changes.
Answers: 1
You know the right answer?
Standardization of a Borax solution (Na2B4O7). You are given a 1.044 M solution of H2SO4. It takes 2...
Questions


Computers and Technology, 24.02.2021 01:00


Mathematics, 24.02.2021 01:00

Chemistry, 24.02.2021 01:00




English, 24.02.2021 01:00

Mathematics, 24.02.2021 01:00




Mathematics, 24.02.2021 01:00


Mathematics, 24.02.2021 01:00

English, 24.02.2021 01:00


Chemistry, 24.02.2021 01:00

Mathematics, 24.02.2021 01:00

 ....(1)
....(1) are the n-factor, concentration and volume of sulfuric acid
are the n-factor, concentration and volume of sulfuric acid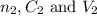 are the n-factor, concentration and volume of borax solution.
are the n-factor, concentration and volume of borax solution.