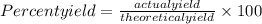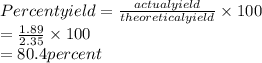Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:30
When the speed of the bottle is 2 m/s, the average maximum height of the beanbag is m.
Answers: 2


Chemistry, 22.06.2019 16:50
What is conserved in the reaction shown below? h2(g) + cl2 (g) --> 2hcl(g)a. mass onlyb. mass and moles onlyc. mass, moles, and molecules onlyd. mass, moles, molecules, and volume
Answers: 2

Chemistry, 22.06.2019 18:50
Asample of tin (ii) chloride has a mass of 0.49 g. after heating, it has a mass of 0.41 g. what is the percent by mass of water in the hydrate? %
Answers: 1
You know the right answer?
A student dissolved 3.50g of copper (II) nitrate in water and mixed it with a solution of sodium car...
Questions

History, 09.09.2021 22:00



English, 09.09.2021 22:00

Business, 09.09.2021 22:00



History, 09.09.2021 22:00






English, 09.09.2021 22:00


Mathematics, 09.09.2021 22:00

History, 09.09.2021 22:00

Geography, 09.09.2021 22:00


History, 09.09.2021 22:00