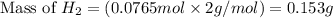Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 00:00
Which type of bonding involves the complete transfer of a valence electron from a less electrogrative atom to a more electronegative one
Answers: 1

Chemistry, 22.06.2019 12:00
Give the set of reactants (including an alkyl halide and a nucleophile) that could be used to synthesize the following ether: draw the molecules on the canvas by choosing buttons from the tools (for bonds and charges), atoms, and templates toolbars, including charges where needed. ch3ch2och2ch2chch3 | ch3
Answers: 1

Chemistry, 22.06.2019 14:30
How can carbon move from "land" to bodies of water? describe the way human impact has lead to increased levels of co2 in the atmosphere.
Answers: 2
You know the right answer?
if 5.0g zinc reacts with 10.0 g hci to produce h2 gas and znci2 according to the following equation...
Questions



Mathematics, 31.07.2019 12:20

Mathematics, 31.07.2019 12:20




Biology, 31.07.2019 12:20


Mathematics, 31.07.2019 12:20



Business, 31.07.2019 12:20

Biology, 31.07.2019 12:20


Mathematics, 31.07.2019 12:20

Business, 31.07.2019 12:20



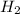 produced is 0.153 g
produced is 0.153 g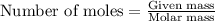 ......(1)
......(1)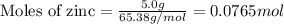
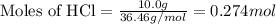

 of HCl
of HCl of
of