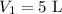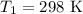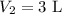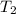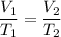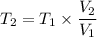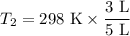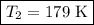Chemistry, 30.06.2021 09:30 papasully1
Imagine you have a 5L balloon that you would like to fit into a 3L container. The current temperature is 298 K. At what temperature would you need to change the system to allow the balloon to fit into the container? Show your work.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:00
Which describes interactions between substances and stomata during photosynthesis? check all that apply. oxygen enters stomata. oxygen is released through stomata. carbon dioxide enters stomata. carbon dioxide is released through stomata. hydrogen enters stomata. hydrogen is released through stomata.
Answers: 1

Chemistry, 22.06.2019 08:30
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 1

Chemistry, 22.06.2019 17:30
In a heat of an engine, if 700 j enters the system, and the piston does 400 j of work what is the final internal (thermal) energy of the system if the initial energy is 1,500 j
Answers: 2

Chemistry, 22.06.2019 20:10
What would happen to a volleyball left outside in the winter? o o o o a. it would expand. b. it would lose air. c. it would shrink. d. it would explode.
Answers: 2
You know the right answer?
Imagine you have a 5L balloon that you would like to fit into a 3L container. The current temperatur...
Questions

History, 20.09.2021 06:00

English, 20.09.2021 06:00

Physics, 20.09.2021 06:00

Chemistry, 20.09.2021 06:00

Mathematics, 20.09.2021 06:00

Mathematics, 20.09.2021 06:00

Mathematics, 20.09.2021 06:00


English, 20.09.2021 06:00

English, 20.09.2021 06:00






Mathematics, 20.09.2021 06:10

Mathematics, 20.09.2021 06:10


Mathematics, 20.09.2021 06:10

Mathematics, 20.09.2021 06:10