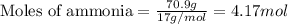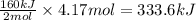2NH3(g)→N2(g)+3H2(g)

Chemistry, 01.07.2021 15:40 rowdycar313p0ao5k
A chemist measures the energy change
ΔH during the following reaction:
2NH3(g)→N2(g)+3H2(g)
ΔH=160kJUse the information to answer the following questions. This reaction is:.
a. endothermic
b. exothermic
Suppose 70.9 g of NH3 react. Will any heat be released or absorbed?
a. Yes, absorbed
b. Yes, released
c. No.
If you said heat will be released or absorbed in the second part of this question, calculate how much heat will be released or absorbed. Round your answer to 3 significant digits.

Answers: 3


Another question on Chemistry




Chemistry, 22.06.2019 07:00
Indicate whether the specified alkyl halides will form primarily substitution products, only elimination products, both substitution and elimination products, or no products when they react with sodium methoxide. 1-bromobutane 1-bromo-2-methylpropane 2-bromobutane 2-bromo-2-methylpropane
Answers: 2
You know the right answer?
A chemist measures the energy change
ΔH during the following reaction:
2NH3(g)→N2(g)+3H2(g)
2NH3(g)→N2(g)+3H2(g)
Questions


Arts, 16.12.2020 18:40





Physics, 16.12.2020 18:40

Mathematics, 16.12.2020 18:40

Mathematics, 16.12.2020 18:40

Spanish, 16.12.2020 18:40


Physics, 16.12.2020 18:40

Mathematics, 16.12.2020 18:40



Spanish, 16.12.2020 18:40


English, 16.12.2020 18:40

Mathematics, 16.12.2020 18:40

Arts, 16.12.2020 18:40

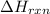 is positive for these reactions.
is positive for these reactions.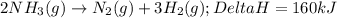
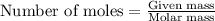 ......(1)
......(1)