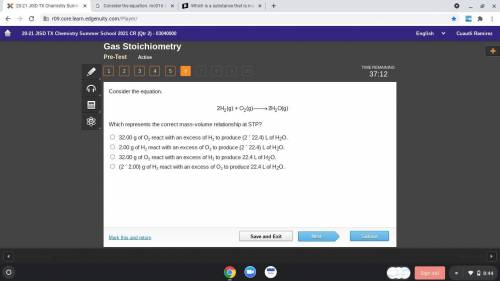
Consider the equation.
Which represents the correct mass-volume relationship at STP?
32.00 g...

Chemistry, 01.07.2021 17:00 juliannabartra
Consider the equation.
Which represents the correct mass-volume relationship at STP?
32.00 g of O2 react with an excess of H2 to produce (2 ´ 22.4) L of H2O.
2.00 g of H2 react with an excess of O2 to produce (2 ´ 22.4) L of H2O.
32.00 g of O2 react with an excess of H2 to produce 22.4 L of H2O.
(2 ´ 2.00) g of H2 react with an excess of O2 to produce 22.4 L of H2O.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:00
Oxidation-reduction reactions (often called "redox" for short) are reactions that involve the transfer of electrons from one species to another. oxidation states, or oxidation numbers, allow chemists to keep track of these electron transfers. in general, one element will lose electrons (oxidation), with the result that it will increase in oxidation number, and another element will gain electrons (reduction), thereby decreasing in oxidation number. the species that is oxidized is called the reducing agent or reductant. the species that is reduced is called the oxidizing agent or oxidant. to sum up: oxidation = increase in oxidation state = loss of electrons = reducing agent reduction = decrease in oxidation state = gain of electrons = oxidizing agent part a which element is oxidized in this reaction? fe2o3+3co→2fe+3co2 enter the elemental symbol. view available hint(s) is oxidized part b which element is reduced in this reaction? 2hcl+2kmno4+3h2c2o4→6co2+2mno2+2kcl+4h2o enter the elemental symbol. view available hint(s) is reduced
Answers: 1

Chemistry, 22.06.2019 07:30
Using data from seismic waves, geologists have learned that earth’s interior is made up of several
Answers: 1

Chemistry, 22.06.2019 12:00
What does a complete balanced chemical equation include? a. exothermic coefficients b. endothermic coefficients c. valence electrons d. molar coefficients
Answers: 1

Chemistry, 22.06.2019 12:20
Which is an example of the practical pursuit of alchemy? a. forming perfect substances. b. transforming base metals. c. developing metalworking techniques. d. linking spiritual characteristics with material substances.
Answers: 1
You know the right answer?
Questions



Arts, 18.12.2020 17:30




Computers and Technology, 18.12.2020 17:30

Mathematics, 18.12.2020 17:30





Social Studies, 18.12.2020 17:30

Advanced Placement (AP), 18.12.2020 17:30


Mathematics, 18.12.2020 17:30



Mathematics, 18.12.2020 17:30



