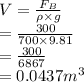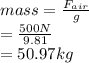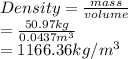Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Turbo the snail moves across the ground at a pace of 12 feet per day. if the garden is 48 feet away, how many days will it take for the snail to get there?
Answers: 2

Chemistry, 22.06.2019 05:30
Which other elements contain the same number of outer electrons as sodium
Answers: 3

Chemistry, 22.06.2019 05:50
What are the 4 phases of matter in order of increasing engery content?
Answers: 2

Chemistry, 22.06.2019 06:00
Oxidation-reduction reactions (often called "redox" for short) are reactions that involve the transfer of electrons from one species to another. oxidation states, or oxidation numbers, allow chemists to keep track of these electron transfers. in general, one element will lose electrons (oxidation), with the result that it will increase in oxidation number, and another element will gain electrons (reduction), thereby decreasing in oxidation number. the species that is oxidized is called the reducing agent or reductant. the species that is reduced is called the oxidizing agent or oxidant. to sum up: oxidation = increase in oxidation state = loss of electrons = reducing agent reduction = decrease in oxidation state = gain of electrons = oxidizing agent part a which element is oxidized in this reaction? fe2o3+3co→2fe+3co2 enter the elemental symbol. view available hint(s) is oxidized part b which element is reduced in this reaction? 2hcl+2kmno4+3h2c2o4→6co2+2mno2+2kcl+4h2o enter the elemental symbol. view available hint(s) is reduced
Answers: 1
You know the right answer?
a sample of unknown material weighs 500 n in air and 200 n when immesersed in alcholol with a specfi...
Questions

Mathematics, 13.11.2020 06:10

Chemistry, 13.11.2020 06:10

Health, 13.11.2020 06:10


History, 13.11.2020 06:10

Mathematics, 13.11.2020 06:10

Mathematics, 13.11.2020 06:10

Biology, 13.11.2020 06:10


Mathematics, 13.11.2020 06:10



Biology, 13.11.2020 06:10



Mathematics, 13.11.2020 06:10




Mathematics, 13.11.2020 06:10

 .
.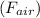 = 500 N
= 500 N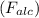 = 200 N
= 200 N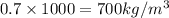
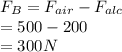
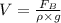
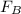 = Buoyant force
= Buoyant force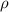 = specific gravity
= specific gravity