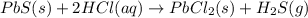Chemistry, 01.07.2021 21:10 cristinaledford3696
Solid lead(II) sulfide reacts with aqueous hydrochloric acid to form solid lead(II) chloride and dihydrogen sulfide gas. Express your answer as a chemical equation.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 10:50
How many liters of oxygen gas, at standard temperature and pressure, will react with 35.8 grams of iron metal? 4 fe (s) + 3 o₂ (g) → 2 fe₂o₃ (s)
Answers: 2

Chemistry, 22.06.2019 11:20
Sodium nitrite (nano2) reacted with 2−iodooctane to give a mixture of two constitutionally isomeric compounds of molecular formula c8h17no2 in a combined yield of 88%. draw reasonable structures for these two isomers. click the "draw structure" button to launch the drawing utility. place the two compounds in the appropriate boxes below.
Answers: 1

Chemistry, 22.06.2019 12:00
Marcel just purchased 1.69 grams of iron fillings in order to make living putty for his 6 year old niece. how many moles of iron are made in his sample?
Answers: 1

Chemistry, 22.06.2019 20:30
Draw a line graph showing the relationship between temperature in kelvin as a function of kinetic energy.
Answers: 3
You know the right answer?
Solid lead(II) sulfide reacts with aqueous hydrochloric acid to form solid lead(II) chloride and dih...
Questions

Business, 13.11.2020 04:10



Mathematics, 13.11.2020 04:10


Mathematics, 13.11.2020 04:10

History, 13.11.2020 04:10

Business, 13.11.2020 04:10


Mathematics, 13.11.2020 04:10

Chemistry, 13.11.2020 04:10





Mathematics, 13.11.2020 04:10



Computers and Technology, 13.11.2020 04:10

Mathematics, 13.11.2020 04:10