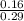Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:30
Compare and contrast the bohr model and the electron cloud models of the atom.
Answers: 1

Chemistry, 22.06.2019 13:50
Amap that uses a range of colors and shading to represent the elevation, depth, or landscape of specific features on earth is a/an map.
Answers: 3

Chemistry, 22.06.2019 19:00
Which statement best describes what happens when molecular compounds melt
Answers: 1

Chemistry, 22.06.2019 21:30
Harry lives in a city, and he has a lung condition known as asthma. on certain days, harry has to stay inside because pollutants in the air make it difficult for him to breathe. which of these pollution sources are nonpoint sources that might bother harry if he goes outside? choose the two that apply.
Answers: 3
You know the right answer?
An aqueous solution contains 0.29 M of benzoic acid (HA) and 0.16 M of sodium benzoate (A-). If the...
Questions

Mathematics, 05.05.2020 09:32

English, 05.05.2020 09:32


Mathematics, 05.05.2020 09:32

Mathematics, 05.05.2020 09:32


Mathematics, 05.05.2020 09:32






Mathematics, 05.05.2020 09:32




English, 05.05.2020 09:32



![\frac{[A^-]}{[HA]}](/tpl/images/1388/3192/1d212.png)