Chemistry, 02.07.2021 22:50 shimonypuck28
A buffer is prepared containing 0.75 M NH3 and 0.20 M NH4 . Calculate the pH of the buffer using the Kb for NH3. g

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:40
Calculate: select the worksheet tab. this tab you calculate the analyte concentration. fill in the first set of boxes ("moles h2so4" and "moles naoh") based on the coefficients in the balanced equation. (if there is no coefficient, the value is 1.) record the appropriate volumes in the "ml naoh" and "ml h2so4" boxes. record the concentration of the titrant in the m naoh box. click calculate. what is the concentration listed
Answers: 2

Chemistry, 22.06.2019 15:00
Which theory was contradicted by experiments with the photoelectric effect? light spreads out after it passes through a small opening. as soon as light strikes metal, electrons will be ejected. visible light, regardless of color, will cause the ejection of electrons when striking metal. the kinetic energy of ejected electrons depends on the frequency of light that strikes the metal.
Answers: 2

Chemistry, 23.06.2019 03:30
Select the correct lewis structure for fluorine which is group 7a element?
Answers: 1

Chemistry, 23.06.2019 03:50
What is the equation fort the alkaline zinc/manganese dioxide cell. a) anode b)cathode c)overall equations.
Answers: 2
You know the right answer?
A buffer is prepared containing 0.75 M NH3 and 0.20 M NH4 . Calculate the pH of the buffer using the...
Questions

Mathematics, 16.09.2021 03:00





Mathematics, 16.09.2021 03:00


Mathematics, 16.09.2021 03:00

Arts, 16.09.2021 03:00

Biology, 16.09.2021 03:00


History, 16.09.2021 03:00



Mathematics, 16.09.2021 03:00

Mathematics, 16.09.2021 03:00

Mathematics, 16.09.2021 03:00

Mathematics, 16.09.2021 03:00

Mathematics, 16.09.2021 03:00

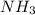
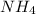
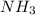
![pOH=pka+log\frac{[salt]}{[base]}\\](/tpl/images/1388/4153/f91b6.png)