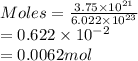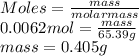Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 20:30
Calculate the percent composition by mass of each element in al(oh)3. use at least three significant figures.
Answers: 1

Chemistry, 23.06.2019 00:30
What is the percent by mass of magnesium sulfate in mgso4.7h2o
Answers: 3

Chemistry, 23.06.2019 05:00
What is dhmo? hint: you find it everywhere something is wet..
Answers: 1
You know the right answer?
Determine the mass in grams of 3.75 x 10^21 atoms of zinc. (the mass of one mole of zinc is 65.39 g)...
Questions

Mathematics, 18.05.2020 09:57


Chemistry, 18.05.2020 09:57

Health, 18.05.2020 09:57



Mathematics, 18.05.2020 09:57



Advanced Placement (AP), 18.05.2020 09:57

Mathematics, 18.05.2020 09:57



Health, 18.05.2020 09:57




Mathematics, 18.05.2020 09:57


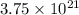 atoms of zinc is 0.405 g.
atoms of zinc is 0.405 g.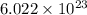 atoms. So, the number of moles in given number of atoms is as follows.
atoms. So, the number of moles in given number of atoms is as follows.