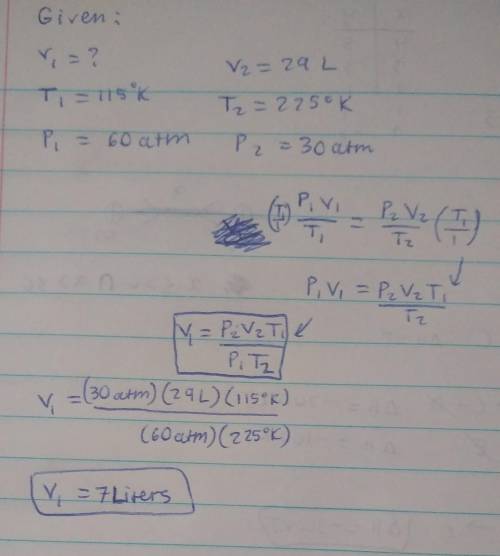Chemistry, 05.07.2021 20:10 alyssalefeber
I have an unknown volume of gas held at a temperature of 115 K in a container with a pressure of 60atm. If by increasing the temperature to 225 K and decreasing the pressure to 30. atm causes the volume of the gas to be 29 liters, how many liters of gas did I start with? SHOW YOUR WORK

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:40
Kc = 0.040 for the system below at 450oc. if a reaction is initiated with 0.40 mole of cl2 and 0.40 mole of pcl3 in a 2.0 liter container, what is the equilibrium concentration of cl2 in the same system? pcl5(g) ⇄ pcl3(g) + cl2(g)
Answers: 3


Chemistry, 22.06.2019 14:00
Calculate the frequency of a wave in a spring toy. the wave has a speed of 1.1 meters per second and a wavelength of 0.1 meters. *
Answers: 2

You know the right answer?
I have an unknown volume of gas held at a temperature of 115 K in a container with a pressure of 60a...
Questions

Mathematics, 07.06.2020 05:58

Mathematics, 07.06.2020 05:58

Mathematics, 07.06.2020 05:58



Mathematics, 07.06.2020 05:58

Mathematics, 07.06.2020 05:58




Mathematics, 07.06.2020 05:58





Mathematics, 07.06.2020 05:58



History, 07.06.2020 05:58

English, 07.06.2020 05:58