
Chemistry, 06.07.2021 09:00 FailingstudentXD
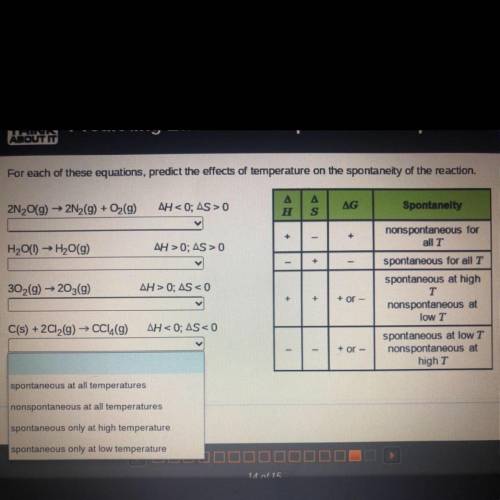
For each of these equations, predict the effects of temperature on the spontaneity of the reaction

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:20
Explain that newton first law,second law and third law of motion?
Answers: 2

Chemistry, 22.06.2019 16:00
What rule is used to determine how many covalent bonds an element can form? a. the number of covalent bonds is equal to six c the number of covalent bonds is equal to five minus the group number plus the group number b. the number of covalent bonds is equal to eight d. none of the above minus the group number select the best answer from the choices provided
Answers: 2


You know the right answer?
For each of these equations, predict the effects of temperature on the spontaneity of the reaction
<...
Questions

Mathematics, 01.02.2020 15:44



Business, 01.02.2020 15:44


English, 01.02.2020 15:44

Physics, 01.02.2020 15:44

Biology, 01.02.2020 15:44

History, 01.02.2020 15:44

Geography, 01.02.2020 15:44


Biology, 01.02.2020 15:44


Mathematics, 01.02.2020 15:44

Biology, 01.02.2020 15:44


Arts, 01.02.2020 15:44

Biology, 01.02.2020 15:44


Mathematics, 01.02.2020 15:44




