Using the balanced equation below,
how many grams of sodium
thiosulfate would be required to<...

Chemistry, 06.07.2021 19:00 destinyranson
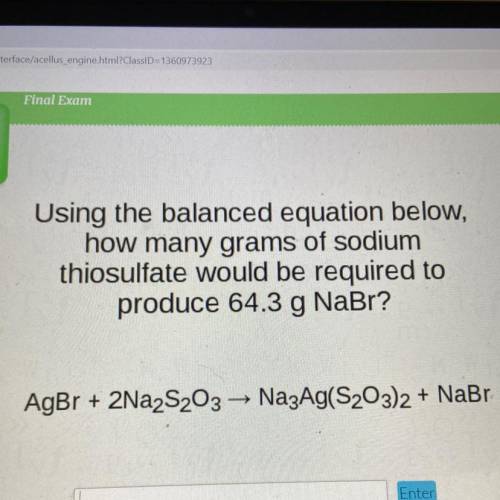
Using the balanced equation below,
how many grams of sodium
thiosulfate would be required to
produce 64.3 g NaBr?
AgBr + 2Na2S203 — Na3Ag(S203)2 + NaBr

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:00
Which atom or ion is the largest? a. k b. k+ c. ca d. ca2+ e. li
Answers: 1

Chemistry, 23.06.2019 00:30
On the periodic table, elements are arranged by which of the following. a. mass numbers. b. increasing atomic number. c. alphabetical order. or d. density
Answers: 1

Chemistry, 23.06.2019 07:00
Scuba divers use tanks of compressed air to them breathe. gases can be compressed because?
Answers: 1

Chemistry, 23.06.2019 07:20
F1.5 mol of nabh4 react, how many moles of b2h6 are formed? 2 nabh4(aq) + h2so4(aq) → 2 h2(g) + na2so4(aq) + b2h6(g)
Answers: 1
You know the right answer?
Questions

Mathematics, 02.12.2020 06:00

Mathematics, 02.12.2020 06:00









Mathematics, 02.12.2020 06:00

Physics, 02.12.2020 06:00

Mathematics, 02.12.2020 06:00

Spanish, 02.12.2020 06:00




Mathematics, 02.12.2020 06:00

Mathematics, 02.12.2020 06:00



