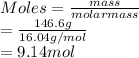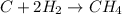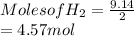Chemistry, 06.07.2021 20:30 ddavid9361
I need help solving this!
For the reaction C + 2H2 → CH4, how many moles of hydrogen are needed to make 146.6 grams of methane, CH4 ?
Round your answer to the nearest tenth. If you answer is a whole number like 4, report the answer as 4.0
Use the following molar masses. If you do not use these masses, the computer will mark your answer incorrect.:
Element
Molar Mass
Hydrogen
1
Carbon
12

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 12:30
Sodium sulfate dissolves as follows: na2so4(s) → 2na+(aq) + so42- (aq). how many moles of na2so4 are required to make 1.0 l of solution in which the na concentration is 0.10 m?
Answers: 2

Chemistry, 22.06.2019 17:30
I'm learning about the periodic tables and what each subject's configuration is. for example, hydrogen is 1s^1, but i don't understand how you get that. can someone me understand how to figure out how to figure this out? sorry if the question makes no sense, but it would really a lot if you could me understand! you so much if you can!
Answers: 1

Chemistry, 22.06.2019 17:30
Which scenario is most similar to the type of collision that gas particles have according to kinetic molecular theory
Answers: 1

Chemistry, 22.06.2019 20:30
From the choices provided below, list the reagent(s) in order that will react with cyclopentanone to form the compound shown below.
Answers: 2
You know the right answer?
I need help solving this!
For the reaction C + 2H2 → CH4, how many moles of hydrogen are needed to...
Questions


English, 03.06.2021 22:10

Chemistry, 03.06.2021 22:10

Mathematics, 03.06.2021 22:10

Mathematics, 03.06.2021 22:10

English, 03.06.2021 22:10

Mathematics, 03.06.2021 22:10


Mathematics, 03.06.2021 22:10


Physics, 03.06.2021 22:10


Mathematics, 03.06.2021 22:10


Physics, 03.06.2021 22:10

Biology, 03.06.2021 22:10

Mathematics, 03.06.2021 22:10


Mathematics, 03.06.2021 22:10

Mathematics, 03.06.2021 22:10

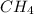 .
.